| Revision as of 23:52, 30 June 2011 editPeryeat (talk | contribs)Extended confirmed users2,689 editsNo edit summary← Previous edit | Latest revision as of 19:12, 2 June 2021 edit undoSdkbBot (talk | contribs)Bots356,562 editsm →Australia: removed erroneous spaceTag: AWB | ||
| (22 intermediate revisions by 14 users not shown) | |||
| Line 1: | Line 1: | ||
| {{chembox | {{chembox | ||
| | Watchedfields = changed | |||
| ⚫ | | verifiedrevid = 437142907 | ||
| ⚫ | | ImageFile = Dimiracetam.svg | ||
| | ImageName = Chemical structure of dimiracetam | |||
| ⚫ | | ImageSize=150px | ||
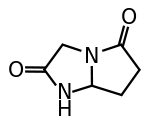
| ⚫ | | IUPACName = (''RS'')-3,6,7,7a-Tetrahydro-1''H''-pyrroloimidazole-2,5-dione | ||
| ⚫ | | OtherNames = | ||
| ⚫ | |Section1={{Chembox Identifiers | ||
| | UNII_Ref = {{fdacite|correct|FDA}} | | UNII_Ref = {{fdacite|correct|FDA}} | ||
| | UNII = 4AW7F70MZO | | UNII = 4AW7F70MZO | ||
| ⚫ | | verifiedrevid = |
||
| ⚫ | | ImageFile = Dimiracetam. |
||
| | imagename = 1 : 1 mixture (racemate) | |||
| ⚫ | | |
||
| ⚫ | | IUPACName = (''RS'')-3,6,7,7a- |
||
| ⚫ | | OtherNames = | ||
| ⚫ | | |
||
| | PubChem = 65955 | | PubChem = 65955 | ||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| Line 21: | Line 22: | ||
| | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChIKey = XTXXOHPHLNROBN-UHFFFAOYSA-N | | StdInChIKey = XTXXOHPHLNROBN-UHFFFAOYSA-N | ||
| | CASNo_Ref = {{cascite|correct|??}} | |||
| | CASNo =126100-97-8 | | CASNo =126100-97-8 | ||
| | ATCCode_prefix = | |||
| | ATCCode_suffix = | |||
| | SMILES= C1CC(=O)N2C1NC(=O)C2 }} | | SMILES= C1CC(=O)N2C1NC(=O)C2 }} | ||
| | |
|Section2={{Chembox Properties | ||
| | Formula = |
| Formula = | ||
| | C=6 | H=8 | N=2 | O=2 | |||
| | MolarMass = 140.140 g/mol | |||
| | Appearance = | | Appearance = | ||
| | Density = | | Density = | ||
| Line 33: | Line 33: | ||
| | BoilingPt = | | BoilingPt = | ||
| | Solubility = }} | | Solubility = }} | ||
| | |
|Section5={{Chembox Pharmacology | ||
| | AdminRoutes = | | AdminRoutes = | ||
| | Bioavail = | | Bioavail = | ||
| Line 43: | Line 43: | ||
| | Legal_US = | | Legal_US = | ||
| | Legal_UK = | | Legal_UK = | ||
| | Legal_AU = | | Legal_AU = S4 | ||
| | Legal_CA = | | Legal_CA = | ||
| | |
| Pregnancy_category = | ||
| | |
| Pregnancy_AU = | ||
| | PregCat_US = }} | |||
| }} | |||
| }} | }} | ||
| '''Dimiracetam''' is a ] drug of the ] family,<ref>{{cite journal | |
'''Dimiracetam''' is a ] drug of the ] family,<ref>{{cite journal | vauthors = Pinza M, Farina C, Cerri A, Pfeiffer U, Riccaboni MT, Banfi S, Biagetti R, Pozzi O, Magnani M, Dorigotti L | display-authors = 6 | title = Synthesis and pharmacological activity of a series of dihydro-1H-pyrroloimidazole-2,5(3H,6H)-diones, a novel class of potent cognition enhancers | journal = Journal of Medicinal Chemistry | volume = 36 | issue = 26 | pages = 4214–20 | date = December 1993 | pmid = 8277504 | doi = 10.1021/jm00078a011 }}</ref> derivatives of which may have application in the treatment of ].<ref>{{cite journal | vauthors = Farina C, Gagliardi S, Ghelardini C, Martinelli M, Norcini M, Parini C, Petrillo P, Ronzoni S | display-authors = 6 | title = Synthesis and biological evaluation of novel dimiracetam derivatives useful for the treatment of neuropathic pain | journal = Bioorganic & Medicinal Chemistry | volume = 16 | issue = 6 | pages = 3224–32 | date = March 2008 | pmid = 18171618 | doi = 10.1016/j.bmc.2007.12.015 }}</ref> | ||
| == |
== Legality == | ||
| === Australia === | |||
| <references/> | |||
| Dimiracetam is a schedule 4 substance in Australia under the ].<ref name="Poisons Stanrard">. comlaw.gov.au</ref> A schedule 4 substance is classified as "Prescription Only Medicine, or Prescription Animal Remedy – Substances, the use or supply of which should be by or on the order of persons permitted by State or Territory legislation to prescribe and should be available from a pharmacist on prescription."<ref name="Poisons Stanrard" /> | |||
| == References == | |||
| {{reflist}} | |||
| {{Racetams}} | {{Racetams}} | ||
| Line 63: | Line 68: | ||
| {{nervous-system-drug-stub}} | {{nervous-system-drug-stub}} | ||
| ] | |||
Latest revision as of 19:12, 2 June 2021
| |
| Names | |
|---|---|
| IUPAC name (RS)-3,6,7,7a-Tetrahydro-1H-pyrroloimidazole-2,5-dione | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C6H8N2O2 |
| Molar mass | 140.142 g·mol |
| Pharmacology | |
| Legal status |
|
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Dimiracetam is a nootropic drug of the racetam family, derivatives of which may have application in the treatment of neuropathic pain.
Legality
Australia
Dimiracetam is a schedule 4 substance in Australia under the Poisons Standard (February 2020). A schedule 4 substance is classified as "Prescription Only Medicine, or Prescription Animal Remedy – Substances, the use or supply of which should be by or on the order of persons permitted by State or Territory legislation to prescribe and should be available from a pharmacist on prescription."
References
- Pinza M, Farina C, Cerri A, Pfeiffer U, Riccaboni MT, Banfi S, et al. (December 1993). "Synthesis and pharmacological activity of a series of dihydro-1H-pyrroloimidazole-2,5(3H,6H)-diones, a novel class of potent cognition enhancers". Journal of Medicinal Chemistry. 36 (26): 4214–20. doi:10.1021/jm00078a011. PMID 8277504.
- Farina C, Gagliardi S, Ghelardini C, Martinelli M, Norcini M, Parini C, et al. (March 2008). "Synthesis and biological evaluation of novel dimiracetam derivatives useful for the treatment of neuropathic pain". Bioorganic & Medicinal Chemistry. 16 (6): 3224–32. doi:10.1016/j.bmc.2007.12.015. PMID 18171618.
- ^ Poisons Standard February 2020. comlaw.gov.au
| Racetams | |
|---|---|
| Racetams |
|
| Phenylpiracetams |
|
| Racetam-like |
|
This drug article relating to the nervous system is a stub. You can help Misplaced Pages by expanding it. |