| Revision as of 01:59, 17 August 2011 editCheMoBot (talk | contribs)Bots141,565 edits Updating {{drugbox}} (no changed fields - added verified revid - updated 'ChemSpiderID_Ref', 'DrugBank_Ref', 'ChEMBL_Ref', 'ChEBI_Ref', 'KEGG_Ref', 'StdInChI_Ref', 'StdInChIKey_Ref', 'ChEBI_Ref') per [[Misplaced Pages:WikiProject Chemicals/Chembox validati← Previous edit | Latest revision as of 20:53, 31 July 2024 edit undoJWBE (talk | contribs)Extended confirmed users10,127 edits added Category:Cyclohexyl compounds using HotCat | ||
| (15 intermediate revisions by 12 users not shown) | |||
| Line 1: | Line 1: | ||
| {{Short description|Chemical compound}} | |||
| {{drugbox | |||
| {{Drugbox | |||
| | Verifiedfields = changed | |||
| | Watchedfields = changed | |||
| ⚫ | | verifiedrevid = 445252268 | ||
| ⚫ | | IUPAC_name = 2-(azepan-1-yl)ethyl 2-cyclohexyl-2-(thiophen-3-yl)acetate | ||
| ⚫ | | image = Cetiedil_small.png | ||
| | image2 = Cetiedil-3D-balls.png | |||
| <!--Clinical data--> | |||
| | tradename = | |||
| ⚫ | | pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> | ||
| ⚫ | | pregnancy_US = <!-- A / B / C / D / X --> | ||
| ⚫ | | pregnancy_category = | ||
| ⚫ | | legal_AU = <!-- S2, S3, S4, S5, S6, S7, S8, S9 or Unscheduled--> | ||
| ⚫ | | legal_CA = <!-- Schedule I, II, III, IV, V, VI, VII, VIII --> | ||
| ⚫ | | legal_UK = <!-- GSL, P, POM, CD, or Class A, B, C --> | ||
| ⚫ | | legal_US = <!-- OTC / Rx-only / Schedule I, II, III, IV, V --> | ||
| ⚫ | | legal_status = | ||
| ⚫ | | routes_of_administration = | ||
| <!--Pharmacokinetic data--> | |||
| ⚫ | | bioavailability = | ||
| ⚫ | | protein_bound = | ||
| ⚫ | | metabolism = | ||
| ⚫ | | elimination_half-life = | ||
| ⚫ | | excretion = | ||
| <!--Identifiers--> | |||
| | CAS_number_Ref = {{cascite|correct|??}} | |||
| ⚫ | | CAS_number = 14176-10-4 | ||
| ⚫ | | ATC_prefix = C04 | ||
| ⚫ | | ATC_suffix = AX26 | ||
| ⚫ | | PubChem = 66384 | ||
| | ChEMBL_Ref = {{ebicite|changed|EBI}} | |||
| | ChEMBL = 419380 | |||
| ⚫ | | DrugBank_Ref = {{drugbankcite|correct|drugbank}} | ||
| ⚫ | | DrugBank = | ||
| | UNII_Ref = {{fdacite|correct|FDA}} | | UNII_Ref = {{fdacite|correct|FDA}} | ||
| | UNII = 621RT200TO | | UNII = 621RT200TO | ||
| | ChemSpiderID_Ref = {{chemspidercite|changed|chemspider}} | |||
| ⚫ | | verifiedrevid = |
||
| | ChemSpiderID = 59759 | |||
| ⚫ | | IUPAC_name |
||
| | smiles = O=C(OCCN1CCCCCC1)C(c2ccsc2)C3CCCCC3 | |||
| ⚫ | | image |
||
| | StdInChI_Ref = {{stdinchicite|changed|chemspider}} | |||
| ⚫ | | CAS_number |
||
| | StdInChI = 1S/C20H31NO2S/c22-20(23-14-13-21-11-6-1-2-7-12-21)19(18-10-15-24-16-18)17-8-4-3-5-9-17/h10,15-17,19H,1-9,11-14H2 | |||
| ⚫ | | ATC_prefix |
||
| | StdInChIKey_Ref = {{stdinchicite|changed|chemspider}} | |||
| ⚫ | | ATC_suffix |
||
| | StdInChIKey = MMNICIJVQJJHHF-UHFFFAOYSA-N | |||
| ⚫ | | PubChem |
||
| ⚫ | | DrugBank_Ref = {{drugbankcite|correct|drugbank}} | ||
| <!--Chemical data--> | |||
| ⚫ | | DrugBank |
||
| | C=20|H=31|N=1|O=2|S=1 | | C=20 | H=31 | N=1 | O=2 | S=1 | ||
| | molecular_weight = 349.53064 | |||
| ⚫ | | bioavailability |
||
| ⚫ | | protein_bound |
||
| ⚫ | | metabolism |
||
| ⚫ | | elimination_half-life = | ||
| ⚫ | | excretion |
||
| ⚫ | | pregnancy_AU |
||
| ⚫ | | pregnancy_US |
||
| ⚫ | | pregnancy_category= | ||
| ⚫ | | legal_AU = |
||
| ⚫ | | legal_CA = |
||
| ⚫ | | legal_UK = |
||
| ⚫ | | legal_US = |
||
| ⚫ | | legal_status |
||
| ⚫ | | routes_of_administration = | ||
| }} | }} | ||
| '''Cetiedil''' is a ] and an anti-sickling agent.<ref name="Alavi_1984">{{cite journal | vauthors = Alavi JB | title = Sickle cell anemia. Pathophysiology and treatment | journal = The Medical Clinics of North America | volume = 68 | issue = 3 | pages = 545–56 | date = May 1984 | pmid = 6205230 | doi = 10.1016/s0025-7125(16)31115-4 }}</ref> | |||
| '''Cetiedil''' is a ] and an anti-sickling agent. | |||
| ==Synthesis== | |||
| Original patents:<ref>Pons, Robba, {{Cite patent|FR|1460571}} and Pons et al., {{Cite patent|FR|M5504}} (1966, 1967, both to ]), C.A. 68, 59429d (1968); 71, 91286c (1969).</ref> Prepn and activity:<ref>Robba, LeGuen, Chim. Ther. 2, 120 (1967).</ref> Revised synthesis:<ref>Roxburgh, Craig J.; Ganellin, C. Robin; Shiner, Mark A. R.; Benton, David C. H.; Dunn, Philip M.; Ayalew, Yeshi; Jenkinson, Donald H. (1996). "The Synthesis and Some Pharmacological Actions of the Enantiomers of the K+-Channel Blocker Cetiedil". Journal of Pharmacy and Pharmacology. 48 (8): 851–859. doi:10.1111/j.2042-7158.1996.tb03986.x.</ref><ref>Charles Pigerol, et al. {{US patent|4108865}} (1978 to Labaz SA).</ref> Analogues:<ref>Roxburgh, Craig J.; Ganellin, C. Robin; Athmani, Salah; Bisi, Alessandra; Quaglia, Wilma; Benton, David C. H.; Shiner, Mark A. R.; Malik-Hall, Misbah; Haylett, Dennis G.; Jenkinson, Donald H. (2001). "Synthesis and Structure−Activity Relationships of Cetiedil Analogues as Blockers of the Ca2+-Activated K+Permeability of Erythrocytes†". Journal of Medicinal Chemistry. 44 (20): 3244–3253. doi:10.1021/jm001113w.</ref>]] | |||
| The Clemmensen reduction of 3-thienylcyclohexyl-glycolic acid, ('''1''') gives cyclohexyl(thiophen-3-yl)acetic acid ('''2'''). Esterification of the sodium salt of the resulting acid with 1-(2-chloroethyl)azepane ('''3''') produces ''cetiedil'' ('''4'''). | |||
| == References == | |||
| {{Reflist}} | |||
| {{Peripheral vasodilators}} | {{Peripheral vasodilators}} | ||
| Line 35: | Line 62: | ||
| ] | ] | ||
| ] | ] | ||
| ] | |||
Latest revision as of 20:53, 31 July 2024
Chemical compound Pharmaceutical compound | |
 | |
| Clinical data | |
|---|---|
| ATC code | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.034.556 |
| Chemical and physical data | |
| Formula | C20H31NO2S |
| Molar mass | 349.53 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (what is this?) (verify) | |
Cetiedil is a vasodilator and an anti-sickling agent.
Synthesis
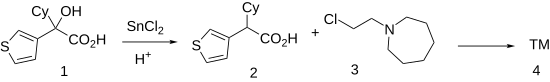
The Clemmensen reduction of 3-thienylcyclohexyl-glycolic acid, CID:11064522 (1) gives cyclohexyl(thiophen-3-yl)acetic acid (2). Esterification of the sodium salt of the resulting acid with 1-(2-chloroethyl)azepane (3) produces cetiedil (4).
References
- Alavi JB (May 1984). "Sickle cell anemia. Pathophysiology and treatment". The Medical Clinics of North America. 68 (3): 545–56. doi:10.1016/s0025-7125(16)31115-4. PMID 6205230.
- Pons, Robba, FR 1460571 and Pons et al., FR M5504 (1966, 1967, both to Innothera), C.A. 68, 59429d (1968); 71, 91286c (1969).
- Robba, LeGuen, Chim. Ther. 2, 120 (1967).
- Roxburgh, Craig J.; Ganellin, C. Robin; Shiner, Mark A. R.; Benton, David C. H.; Dunn, Philip M.; Ayalew, Yeshi; Jenkinson, Donald H. (1996). "The Synthesis and Some Pharmacological Actions of the Enantiomers of the K+-Channel Blocker Cetiedil". Journal of Pharmacy and Pharmacology. 48 (8): 851–859. doi:10.1111/j.2042-7158.1996.tb03986.x.
- Charles Pigerol, et al. U.S. patent 4,108,865 (1978 to Labaz SA).
- Roxburgh, Craig J.; Ganellin, C. Robin; Athmani, Salah; Bisi, Alessandra; Quaglia, Wilma; Benton, David C. H.; Shiner, Mark A. R.; Malik-Hall, Misbah; Haylett, Dennis G.; Jenkinson, Donald H. (2001). "Synthesis and Structure−Activity Relationships of Cetiedil Analogues as Blockers of the Ca2+-Activated K+Permeability of Erythrocytes†". Journal of Medicinal Chemistry. 44 (20): 3244–3253. doi:10.1021/jm001113w.
| Peripheral vasodilators (C04) | |
|---|---|
| Phenylethanolamine derivatives | |
| Alpha blockers |
|
| Nicotinic acid and derivatives | |
| Purine derivatives | |
| Ergot alkaloids | |
| Other peripheral vasodilators | |
This drug article relating to the cardiovascular system is a stub. You can help Misplaced Pages by expanding it. |