| Revision as of 12:38, 20 November 2011 editRjwilmsiBot (talk | contribs)Bots, Pending changes reviewers1,602,950 editsm fixing page range dashes using AWB (7859)← Previous edit | Latest revision as of 22:49, 26 August 2024 edit undoCitation bot (talk | contribs)Bots5,436,558 edits Altered url. URLs might have been anonymized. Add: authors 1-1. Removed parameters. Some additions/deletions were parameter name changes. | Use this bot. Report bugs. | #UCB_CommandLine | ||
| (62 intermediate revisions by 44 users not shown) | |||
| Line 1: | Line 1: | ||
| {{chembox | {{chembox | ||
| | Watchedfields = changed | |||
| | verifiedrevid = 455336630 | |||
| | verifiedrevid = 455336630 | |||
| | ImageFile = Diethylzinc structure.svg | |||
| | ImageFile = Diethylzinc structure.svg | |||
| | ImageSize = | |||
| | ImageFile1 = Diethylzinc-3D-balls.png | | ImageFile1 = Diethylzinc-3D-balls.png | ||
| | IUPACName = diethylzinc | | IUPACName = diethylzinc | ||
| | Section1 = {{Chembox Identifiers | |||
| | OtherNames = | |||
| |ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| | Section1 = {{Chembox Identifiers | |||
| |ChemSpiderID = 10413128 | |||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| |InChI = 1/2C2H5.Zn/c2*1-2;/h2*1H2,2H3;/rC4H10Zn/c1-3-5-4-2/h3-4H2,1-2H3 | |||
| | ChemSpiderID = 10413128 | |||
| |InChIKey = HQWPLXHWEZZGKY-GFXTWEBUAS | |||
| | InChI = 1/2C2H5.Zn/c2*1-2;/h2*1H2,2H3;/rC4H10Zn/c1-3-5-4-2/h3-4H2,1-2H3 | |||
| |StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| | InChIKey = HQWPLXHWEZZGKY-GFXTWEBUAS | |||
| |StdInChI = 1S/2C2H5.Zn/c2*1-2;/h2*1H2,2H3; | |||
| | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| |StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| | StdInChI = 1S/2C2H5.Zn/c2*1-2;/h2*1H2,2H3; | |||
| |StdInChIKey = HQWPLXHWEZZGKY-UHFFFAOYSA-N | |||
| | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| |CASNo_Ref = {{cascite|correct|CAS}} | |||
| | StdInChIKey = HQWPLXHWEZZGKY-UHFFFAOYSA-N | |||
| |CASNo = 557-20-0 | |||
| | CASNo_Ref = {{cascite|correct|CAS}} | |||
| |PubChem = 11185 | |||
| | CASNo = 557-20-0 | |||
| |ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| | PubChem = | |||
| |ChEBI = 51496 | |||
| | ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| |EC_number = 209-161-3 | |||
| | ChEBI = 51496 | |||
| |UNNumber = 1366 | |||
| | SMILES = CCCC | |||
| |UNII = S0W5NQH7C6 | |||
| |SMILES = CCCC | |||
| }} | }} | ||
| | Section2 = {{Chembox Properties | | Section2 = {{Chembox Properties | ||
| | |
|Formula = (C<sub>2</sub>H<sub>5</sub>)<sub>2</sub>Zn | ||
| | |
|MolarMass = 123.50 g/mol | ||
| |Density = 1.205 g/mL | |||
| | Appearance = | |||
| |MeltingPtC = -28 | |||
| | Density = 1.205 g/mL | |||
| |BoilingPtC = 117 | |||
| | MeltingPt = -28 °C | |||
| |Solubility = Reacts | |||
| | BoilingPt = 117 °C | |||
| | Solubility = Reacts violently | |||
| }} | |||
| | Section3 = {{Chembox Hazards | |||
| | EUClass = Flammable ('''F'''); Corrosive ('''C'''); Dangerous for the environment ('''N''') | |||
| | MainHazards = | |||
| | FlashPt = | |||
| | Autoignition = | |||
| }} | |||
| }} | }} | ||
| | Section3 = {{Chembox Hazards | |||
| '''Diethylzinc''' (C<sub>2</sub>H<sub>5</sub>)<sub>2</sub>Zn, or DEZn, is a highly ] ] consisting of a zinc center bound to two ]s. This colourless liquid is an important ] in ] and available commercially as a solution in ]s, ], or ]. | |||
| |MainHazards = Flammable and corrosive liquid, pyrophoric in air, may explode in contact with water. | |||
| |NFPA-H = 1 | |||
| |NFPA-F = 4 | |||
| |NFPA-R = 3 | |||
| |NFPA-S = W | |||
| | ExternalSDS = | |||
| |GHSPictograms = {{GHS02}}{{GHS05}}{{GHS09}} | |||
| |GHSSignalWord = Danger | |||
| |HPhrases = {{H-phrases|225|250|260|302+312+332|314|410}} | |||
| |PPhrases = {{P-phrases|210|222|223|231+232|233|240|241|242|243|260|264|273|280|301+330+331|302+334|303+361+353|304+340|305+351+338|310|321|335+334|363|370+378|391|402+404|403+235|405|422|501}} | |||
| }}<ref>{{Cite web|url=http://www.newenv.com/resources/nfpa_chemicals|title = New Environment Inc. - NFPA Chemicals}}</ref> | |||
| }} | |||
| '''Diethylzinc''' (C<sub>2</sub>H<sub>5</sub>)<sub>2</sub>Zn, or DEZ, is a highly ] and reactive ] consisting of a ] center bound to two ]s. This colourless liquid is an important ] in ]. It is available commercially as a solution in ]s, ], or ], or as a pure liquid. | |||
| ==Synthesis== | ==Synthesis== | ||
| ] first reported the compound in 1848 from zinc and ], the first organozinc compound discovered.<ref>{{cite journal | title = On the isolation of the organic radicals | author = ] | journal = Quarterly Journal of the Chemical Society | year = 1850 | volume = 2 | pages = 263–296 | doi = 10.1039/QJ8500200263 | issue = 3| url = https://zenodo.org/record/1861200}}</ref><ref>{{cite journal | author = Dietmar Seyferth | title = Zinc Alkyls, Edward Frankland, and the Beginnings of Main-Group Organometallic Chemistry | journal = ] | year = 2001 | volume = 20 | issue = 14 | pages = 2940–2955 | doi = 10.1021/om010439f| doi-access = }}</ref> He improved the synthesis by using ] as starting material.<ref>{{cite journal | title = On a new reaction for the production of the zinc-compounds of the alkyl-radical | author = E. Frankland, B. F. Duppa | journal = ]| year = 1864 | volume = 17 | pages = 29–36 | doi = 10.1039/JS8641700029| url =https://zenodo.org/record/1767014}}</ref> The contemporary synthesis consists of the reaction of a 1:1 mixture of ethyl iodide and ethyl bromide with a ], a source of reactive zinc.<ref>{{OrgSynth | author = C. R. Noller | title = Diethyl Zinc | collvol = 2 | collvolpages = 184 | year = 1943 | prep = cv2p0184}}</ref> | |||
| ] first reported the compound in 1848 from zinc and ], the first organozinc compound discovered.<ref>{{cite journal | |||
| | title = On the isolation of the organic radicals | |||
| | author = ] | |||
| | journal = Quarterly ] | |||
| | year = 1850 | |||
| | volume = 2 | |||
| | pages = 263 | |||
| | doi = 10.1039/QJ8500200263 | |||
| | issue = 3}}</ref><ref>{{cite journal | author = Dietmar Seyferth | title = Zinc Alkyls, Edward Frankland, and the Beginnings of Main-Group Organometallic Chemistry | journal = ] | year = 2001 | volume = 20 | pages = 2940–2955 | doi = 10.1021/om010439f}}</ref> He improved the synthesis by using diethyl mercury as starting material <ref>{{cite journal | |||
| | title = On a new reaction for the production of the zinc-compounds of the alkyl-radical | |||
| | author = ], B. F. Duppa | |||
| | journal = ] | |||
| | year = 1864 | |||
| | volume = 17 | |||
| | pages = 29–36 | |||
| | doi = 10.1039/JS8641700029}}</ref> The contemporary synthesis consists of the reaction of a 1:1 mixture of ethyl iodide and ethyl bromide with a ], a source of reactive zinc.<ref>{{OrgSynth | author = C. R. Noller | title = Diethyl Zinc | collvol = 2 | collvolpages = 184 | year = 1943 | prep = cv2p0184}}</ref> | |||
| ==Structure== | ==Structure== | ||
| The compound crystallizes in a ] body-centered ] of ] symmetry I4<sub>1</sub>md. In the ] diethylzinc shows nearly linear Zn centres. |
The compound crystallizes in a ] body-centered ] of ] symmetry I4<sub>1</sub>md. In the ] diethylzinc shows nearly linear Zn centres. The Zn-C bonds measure 194.8(5) pm, while the C-Zn-C angle is slightly bent with 176.2(4)°.<ref>{{cite journal |author1=John Bacsa |author2=Felix Hanke |author3=Sarah Hindley |author4=Rajesh Odedra |author5=George R. Darling |author6=Anthony C. Jones |author7=Alexander Steiner | title = The Solid State Structures of Dimethylzinc and Diethylzinc | journal = ] | year = 2011 | volume = 50 |issue=49 | pages = 11685–11687 | doi = 10.1002/anie.201105099|pmc=3326375 | pmid=21919175}}</ref> The structure of the ] shows a very similar Zn-C distance (195.0(2) pm).<ref>{{cite journal |author1=A. Haaland |author2=J. C. Green |author3=G. S. McGrady |author4=A. J. Downs |author5=E. Gullo |author6=M. J. Lyall |author7=J. Timberlake | title = The length, strength and polarity of metal–carbon bonds: dialkylzinc compounds studied by density functional theory calculations, gas electron diffraction and photoelectron spectroscopy | journal = ] |issue=22 | year = 2003 | pages = 4356–4366 | doi = 10.1039/B306840B}}</ref> | ||
| ==Uses== | ==Uses== | ||
| Despite its highly pyrophoric nature, diethylzinc is an important chemical reagent. It is used in ] as a source of the ethyl ] in ]s to ] groups. For example, the ] addition of an ] to ]<ref>{{OrgSynth | author = Masato Kitamura, Hiromasa Oka, Seiji Suga, and ] | title = <nowiki>Catalytic Enantioselective Addition of Dialkylzincs to Aldehydes Using (2S)-(−)-3-exo-(Dimethylamino)isoborneol : (S)-1-Phenyl-1-propanol</nowiki> |
Despite its highly pyrophoric nature, diethylzinc is an important chemical reagent. It is used in ] as a source of the ethyl ] in ]s to ] groups. For example, the ] addition of an ] to ]<ref>{{OrgSynth | author = Masato Kitamura, Hiromasa Oka, Seiji Suga, and ] | title = <nowiki>Catalytic Enantioselective Addition of Dialkylzincs to Aldehydes Using (2S)-(−)-3-exo-(Dimethylamino)isoborneol : (S)-1-Phenyl-1-propanol</nowiki>| collvol = 10 | collvolpages = 635 | year = 2004 | prep = v79p0139}}</ref> and ]s.<ref>{{OrgSynth | author = Jean-Nicolas Desrosiers, Alexandre Côté, Alessandro A. Boezio, and André B. Charette | title = Preparation of Enantiomerically Enriched (1S)-1-Phenylpropan-1-amine Hydrochloride by a Catalytic Addition of Diorganozinc Reagents to Imines | volume = 83 | pages = 5 | year = 2005 | prep = v83p0005}}</ref> | ||
| Additionally, it is commonly used in combination with ] as a ] to convert ] into ] groups.<ref>{{OrgSynth | author = André B. Charette and Hélène Lebel | title = (2S,3S)-(+)-(3-Phenylcyclopropyl)methanol | collvol = 10 | collvolpages = 613 | year = 2004 | prep = v76p0086}}</ref><ref>{{OrgSynth | author = Yoshihiko Ito, Shotaro Fujii, Masashi Nakatuska, Fumio Kawamoto, and Takeo Saegusa | title = One-Carbon Ring Expansion of Cycloalkanones to Conjugated Cycloalkenones: 2-Cyclohepten-1-one | collvol = 6 | collvolpages = 327 | year = 1988 | prep = cv6p0327}}</ref> |
Additionally, it is commonly used in combination with ] as a ] to convert ] into ] groups.<ref>{{OrgSynth | author = André B. Charette and Hélène Lebel | title = (2S,3S)-(+)-(3-Phenylcyclopropyl)methanol | collvol = 10 | collvolpages = 613 | year = 2004 | prep = v76p0086}}</ref><ref>{{OrgSynth | author = Yoshihiko Ito, Shotaro Fujii, Masashi Nakatuska, Fumio Kawamoto, and Takeo Saegusa | title = One-Carbon Ring Expansion of Cycloalkanones to Conjugated Cycloalkenones: 2-Cyclohepten-1-one | collvol = 6 | collvolpages = 327 | year = 1988 | prep = cv6p0327}}</ref> It is less ] than related ] and ]s, so it may be used when a "softer" nucleophile is needed. | ||
| It is also used extensively in ] as a |
It is also used extensively in ] chemistry as a zinc source in the synthesis of ]. Particularly in the formation of the ] shell for core/shell-type ].<ref>{{cite journal | title = CdSe/CdS/ZnS and CdSe/ZnSe/ZnS Core−Shell−Shell Nanocrystals |author1=Dmitri V. Talapin |author2=Ivo Mekis |author3=Stephan Götzinger |author4=Andreas Kornowski |author5=Oliver Benson |author6=Horst Weller† | journal = ] | year = 2004 | volume = 108 | pages = 18826–18831 | doi = 10.1021/jp046481g | issue = 49}}</ref> | ||
| | title = CdSe/CdS/ZnS and CdSe/ZnSe/ZnS Core−Shell−Shell Nanocrystals | |||
| | author = Dmitri V. Talapin, Ivo Mekis, Stephan Götzinger, Andreas Kornowski, Oliver Benson, and Horst Weller† | |||
| | journal = ] | |||
| | year = 2004 | |||
| | volume = 108 | |||
| | pages = 18826–18831 | |||
| | doi = 10.1021/jp046481g | |||
| | issue = 49}}</ref> | |||
| While in ], it can be used as part of the catalyst for a ] reaction, whereby it participates in living polymerization.<ref>{{cite journal | While in ], it can be used as part of the catalyst for a ] reaction, whereby it participates in living polymerization.<ref>{{cite journal | ||
| | title = Hydrogen iodide/zinc iodide: a new initiating system for living cationic polymerization of vinyl ethers at room temperature | | title = Hydrogen iodide/zinc iodide: a new initiating system for living cationic polymerization of vinyl ethers at room temperature | ||
| | |
|author1=Mitsuo Sawamoto |author2=Chihiro Okamoto |author3=Toshinobu Higashimura |journal = ] | year = 1987 | volume = 20 | pages = 2693–2697 | doi = 10.1021/ma00177a010 | issue = 11|bibcode=1987MaMol..20.2693S }}</ref> | ||
| | journal = ] | |||
| | year = 1987 | |||
| | volume = 20 | |||
| | pages = 2693–2697 | |||
| | doi = 10.1021/ma00177a010 | |||
| | issue = 11}}</ref> | |||
| Diethylzinc is not limited to only being used in chemistry. Because of its high reactivity toward air, it was used in small quantities as a ] or "self igniting" liquid rocket fuel<ref name=Clark2018>{{cite book |isbn = 978-0-8135-9918-2 |title = Ignition!: An Informal History of Liquid Rocket Propellants |last1 = Clark |first1 = John Drury |author-link=John Drury Clark |date = 23 May 2018 |publisher = Rutgers University Press |url=https://books.google.com/books?id=BdU4DwAAQBAJ&q=diethyl%20zinc |pages=302 |oclc=281664}}</ref>{{rp|9}}<ref name="Sutton">{{Cite book | last1 = Sutton | first1 = George P. | last2 = Biblarz | first2 = Oscar | title = Rocket Propulsion Elements - Seventh Edition | publisher = John Wiley & Sons, Inc. | year = 2001 | isbn = 0-471-32642-9 |url = http://mae-nas.eng.usu.edu/MAE_5540_Web/propulsion_systems/subpages/Rocket_Propulsion_Elements.pdf | url-status=live | archive-url=https://web.archive.org/web/20220228001253/http://mae-nas.eng.usu.edu/MAE_5540_Web/propulsion_systems/subpages/Rocket_Propulsion_Elements.pdf |archive-date=28 February 2022}}</ref>{{rp|323}}—it ignites on contact with oxidizer, so the rocket motor need only contain a pump, without a spark source for ignition. Diethylzinc was also investigated by the United States ] as a potential means of ] of books printed on wood pulp paper. Diethylzinc vapour would, in theory, neutralize acid residues in the paper, leaving slightly ]ne ] residues. Although initial results were promising, the project was abandoned. A variety of adverse results prevented the method's adoption. Most infamously, the final prototype suffered damage in a series of diethylzinc explosions from trace amounts of water vapor in the chamber. This led the authors of the study to humorously comment:{{blockquote| It has also been established that tight or loose packing of books; the amount of alkaline reserve; reactions of DEZ with degradation products, unknown paper chemicals and adhesives; phases of the moon and the positions of various planets and constellations do not have any influence on the observed adverse effects of DEZ treatment.<ref>{{Citation |author1 = Kenneth E. Harris|author2 = Chandru J. Shahani |year = 2004 |title = Mass Deacidification: An Initiative To Refine The Diethyl Zinc Process |publisher = ] |location = ] |url = https://www.loc.gov/preservation/resources/deacid/dez.pdf |url-status = dead|archiveurl =https://web.archive.org/web/20130514014013/http://www.loc.gov/preservation/resources/deacid/dez.pdf|archivedate = 2013-05-14}}</ref>}} | |||
| In microelectronics, diethylzinc is used as a ].{{Citation needed|date=December 2007}} | |||
| |author= Kenneth E. Harris and Chandru J. Shahani | |||
| |year= 2004 | |||
| For corrosion protection in ] of the ] design, ] is produced by first passing diethylzinc through an ] centrifuge. | |||
| |title= Mass Deacidification: An Initiative To Refine The Diethyl Zinc Process | |||
| |publisher= ] | |||
| The pyrophoricity of diethylzinc can be used to test the inert atmosphere inside a ]. An oxygen concentration of only a few parts per million will cause a bottle of diethylzinc to fume when opened.<ref>{{Cite book|last1=Shriver|first1=Duward F.|title=The Manipulation of Air-Sensitive Compounds|last2=Drezdzon|first2=Mark A.|publisher=John Wiley & Sons|year=1986|isbn=0-471-86773-X|pages=57}}</ref> | |||
| |publication-place= ] | |||
| |url= http://www.loc.gov/preservation/resources/deacid/dez.pdf | |||
| }}</ref> {{quotation | It has also been established that tight or loose packing of books; the amount of alkaline reserve; reactions of DEZ with degradation products, unknown paper chemicals and adhesives; phases of the moon and the positions of various planets and constellations do not have any influence on the observed adverse effects of DEZ treatment.}} | |||
| In microelectronics, diethylzinc is used as a ].{{Fact|date=December 2007}} | |||
| ==Safety== | ==Safety== | ||
| Diethylzinc |
Diethylzinc may explode when mixed with water and can spontaneously ]. It should therefore be handled using ]s. | ||
| ==References== | ==References== | ||
| Line 100: | Line 75: | ||
| ==External links== | ==External links== | ||
| * |
*Demonstration of the ignition of diethylzinc in air | ||
| {{Zinc compounds}} | |||
| ] | ] | ||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 22:49, 26 August 2024
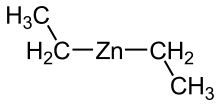
| |
| |
| Names | |
|---|---|
| IUPAC name diethylzinc | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.008.330 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| UN number | 1366 |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | (C2H5)2Zn |
| Molar mass | 123.50 g/mol |
| Density | 1.205 g/mL |
| Melting point | −28 °C (−18 °F; 245 K) |
| Boiling point | 117 °C (243 °F; 390 K) |
| Solubility in water | Reacts |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
| Main hazards | Flammable and corrosive liquid, pyrophoric in air, may explode in contact with water. |
| GHS labelling: | |
| Pictograms |   
|
| Signal word | Danger |
| Hazard statements | H225, H250, H260, H302+H312+H332, H314, H410 |
| Precautionary statements | P210, P222, P223, P231+P232, P233, P240, P241, P242, P243, P260, P264, P273, P280, P301+P330+P331, P302+P334, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P335+P334, P363, P370+P378, P391, P402+P404, P403+P235, P405, P422, P501 |
| NFPA 704 (fire diamond) |
 |
| Safety data sheet (SDS) | External MSDS |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
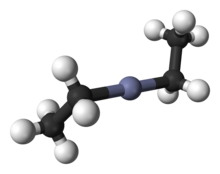
Diethylzinc (C2H5)2Zn, or DEZ, is a highly pyrophoric and reactive organozinc compound consisting of a zinc center bound to two ethyl groups. This colourless liquid is an important reagent in organic chemistry. It is available commercially as a solution in hexanes, heptane, or toluene, or as a pure liquid.
Synthesis
Edward Frankland first reported the compound in 1848 from zinc and ethyl iodide, the first organozinc compound discovered. He improved the synthesis by using diethyl mercury as starting material. The contemporary synthesis consists of the reaction of a 1:1 mixture of ethyl iodide and ethyl bromide with a zinc-copper couple, a source of reactive zinc.
Structure
The compound crystallizes in a tetragonal body-centered unit cell of space group symmetry I41md. In the solid-state diethylzinc shows nearly linear Zn centres. The Zn-C bonds measure 194.8(5) pm, while the C-Zn-C angle is slightly bent with 176.2(4)°. The structure of the gas-phase shows a very similar Zn-C distance (195.0(2) pm).
Uses
Despite its highly pyrophoric nature, diethylzinc is an important chemical reagent. It is used in organic synthesis as a source of the ethyl carbanion in addition reactions to carbonyl groups. For example, the asymmetric addition of an ethyl group to benzaldehyde and imines. Additionally, it is commonly used in combination with diiodomethane as a Simmons-Smith reagent to convert alkenes into cyclopropyl groups. It is less nucleophilic than related alkyllithium and Grignard reagents, so it may be used when a "softer" nucleophile is needed. It is also used extensively in materials science chemistry as a zinc source in the synthesis of nanoparticles. Particularly in the formation of the zinc sulfide shell for core/shell-type quantum dots. While in polymer chemistry, it can be used as part of the catalyst for a chain shuttling polymerization reaction, whereby it participates in living polymerization.
Diethylzinc is not limited to only being used in chemistry. Because of its high reactivity toward air, it was used in small quantities as a hypergolic or "self igniting" liquid rocket fuel—it ignites on contact with oxidizer, so the rocket motor need only contain a pump, without a spark source for ignition. Diethylzinc was also investigated by the United States Library of Congress as a potential means of mass deacidification of books printed on wood pulp paper. Diethylzinc vapour would, in theory, neutralize acid residues in the paper, leaving slightly alkaline zinc oxide residues. Although initial results were promising, the project was abandoned. A variety of adverse results prevented the method's adoption. Most infamously, the final prototype suffered damage in a series of diethylzinc explosions from trace amounts of water vapor in the chamber. This led the authors of the study to humorously comment:
It has also been established that tight or loose packing of books; the amount of alkaline reserve; reactions of DEZ with degradation products, unknown paper chemicals and adhesives; phases of the moon and the positions of various planets and constellations do not have any influence on the observed adverse effects of DEZ treatment.
In microelectronics, diethylzinc is used as a doping agent.
For corrosion protection in nuclear reactors of the light water reactor design, depleted zinc oxide is produced by first passing diethylzinc through an enrichment centrifuge.
The pyrophoricity of diethylzinc can be used to test the inert atmosphere inside a glovebox. An oxygen concentration of only a few parts per million will cause a bottle of diethylzinc to fume when opened.
Safety
Diethylzinc may explode when mixed with water and can spontaneously ignite upon contact with air. It should therefore be handled using air-free techniques.
References
- "New Environment Inc. - NFPA Chemicals".
- E. Frankland (1850). "On the isolation of the organic radicals". Quarterly Journal of the Chemical Society. 2 (3): 263–296. doi:10.1039/QJ8500200263.
- Dietmar Seyferth (2001). "Zinc Alkyls, Edward Frankland, and the Beginnings of Main-Group Organometallic Chemistry". Organometallics. 20 (14): 2940–2955. doi:10.1021/om010439f.
- E. Frankland, B. F. Duppa (1864). "On a new reaction for the production of the zinc-compounds of the alkyl-radical". Journal of the Chemical Society. 17: 29–36. doi:10.1039/JS8641700029.
- C. R. Noller (1943). "Diethyl Zinc". Organic Syntheses; Collected Volumes, vol. 2, p. 184.
- John Bacsa; Felix Hanke; Sarah Hindley; Rajesh Odedra; George R. Darling; Anthony C. Jones; Alexander Steiner (2011). "The Solid State Structures of Dimethylzinc and Diethylzinc". Angewandte Chemie International Edition. 50 (49): 11685–11687. doi:10.1002/anie.201105099. PMC 3326375. PMID 21919175.
- A. Haaland; J. C. Green; G. S. McGrady; A. J. Downs; E. Gullo; M. J. Lyall; J. Timberlake (2003). "The length, strength and polarity of metal–carbon bonds: dialkylzinc compounds studied by density functional theory calculations, gas electron diffraction and photoelectron spectroscopy". Dalton Transactions (22): 4356–4366. doi:10.1039/B306840B.
- Masato Kitamura, Hiromasa Oka, Seiji Suga, and Ryōji Noyori (2004). "Catalytic Enantioselective Addition of Dialkylzincs to Aldehydes Using (2S)-(−)-3-exo-(Dimethylamino)isoborneol : (S)-1-Phenyl-1-propanol". Organic Syntheses
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 10, p. 635. - Jean-Nicolas Desrosiers, Alexandre Côté, Alessandro A. Boezio, and André B. Charette (2005). "Preparation of Enantiomerically Enriched (1S)-1-Phenylpropan-1-amine Hydrochloride by a Catalytic Addition of Diorganozinc Reagents to Imines". Organic Syntheses. 83: 5
{{cite journal}}: CS1 maint: multiple names: authors list (link). - André B. Charette and Hélène Lebel (2004). "(2S,3S)-(+)-(3-Phenylcyclopropyl)methanol". Organic Syntheses; Collected Volumes, vol. 10, p. 613.
- Yoshihiko Ito, Shotaro Fujii, Masashi Nakatuska, Fumio Kawamoto, and Takeo Saegusa (1988). "One-Carbon Ring Expansion of Cycloalkanones to Conjugated Cycloalkenones: 2-Cyclohepten-1-one". Organic Syntheses
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 6, p. 327. - Dmitri V. Talapin; Ivo Mekis; Stephan Götzinger; Andreas Kornowski; Oliver Benson; Horst Weller† (2004). "CdSe/CdS/ZnS and CdSe/ZnSe/ZnS Core−Shell−Shell Nanocrystals". Journal of Physical Chemistry B. 108 (49): 18826–18831. doi:10.1021/jp046481g.
- Mitsuo Sawamoto; Chihiro Okamoto; Toshinobu Higashimura (1987). "Hydrogen iodide/zinc iodide: a new initiating system for living cationic polymerization of vinyl ethers at room temperature". Macromolecules. 20 (11): 2693–2697. Bibcode:1987MaMol..20.2693S. doi:10.1021/ma00177a010.
- Clark, John Drury (23 May 2018). Ignition!: An Informal History of Liquid Rocket Propellants. Rutgers University Press. p. 302. ISBN 978-0-8135-9918-2. OCLC 281664.
- Sutton, George P.; Biblarz, Oscar (2001). Rocket Propulsion Elements - Seventh Edition (PDF). John Wiley & Sons, Inc. ISBN 0-471-32642-9. Archived (PDF) from the original on 28 February 2022.
- Kenneth E. Harris; Chandru J. Shahani (2004), Mass Deacidification: An Initiative To Refine The Diethyl Zinc Process (PDF), Washington, D.C.: Library of Congress, archived from the original (PDF) on 2013-05-14
- Shriver, Duward F.; Drezdzon, Mark A. (1986). The Manipulation of Air-Sensitive Compounds. John Wiley & Sons. p. 57. ISBN 0-471-86773-X.
External links
- Demonstration of the ignition of diethylzinc in air Video - University of Nottingham
| Zinc compounds | |||
|---|---|---|---|
| Zinc(I) |
| ||
| Zinc(II) |
| ||