| |
| Names | |
|---|---|
| Other names Zinc dilactate, zinc 2-hydroxypropionate | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.036.510 |
| EC Number |
|
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C 6H 10ZnO 6 |
| Molar mass | 245.5 |
| Appearance | White crystals |
| Melting point | 277 °C (531 °F; 550 K) |
| Solubility in water | Soluble |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
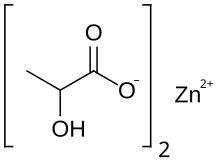
Zinc lactate is a chemical compound, a salt of zinc and lactic acid with the formula Zn(C3H5O3)2.
Synthesis
Reaction of lactic acid with zinc oxide:
- 2CH3CH(OH)COOH + ZnO → Zn(C3H5O3)2 + H2O
Physical properties
Zinc lactate appears as a white to almost white fine powder.
Zinc lactate is nearly odourless, highly soluble in water, and insoluble in ethanol.
Zinc lactate forms dihydrates with the chemical formula Zn(C3H5O3)2 • 2H2O.
Use
The compound is used in dental care products like toothpaste or mouthwash.
Can also be used as a dietary ingredient and as a nutrient.
The compound has antioxidant properties in mammals and can improve intestinal function.
References
- Original Communications. Rumford Press. 1912. p. 356. Retrieved 23 January 2022.
- Benninga, H. (30 June 1990). A History of Lactic Acid Making: A Chapter in the History of Biotechnology. Springer Science & Business Media. p. 132. ISBN 978-0-7923-0625-2. Retrieved 23 January 2022.
- "Zinc Lactate - Jungbunzlauer". Jungbunzlauer. Retrieved 23 January 2022.
- "Clinical Effect of Toothpaste and Mouth Rinse Containing Zinc Lactate on Oral Malodor Reduction - School of Public Health | UAB". University of Alabama at Birmingham School of Public Health. Retrieved 23 January 2022.
- "Jost Chemical - Zinc Lactate Dihydrate Powder, CAS Number 63179-81-7". Jost Chemical Co. Retrieved 23 January 2022.
- Tang, Wenjie; Long, Jing; Li, Tiejun; Yang, Lingyuan; Li, Jianzhong; He, Liuqin; Li, Shuwei; Kuang, Shengyao; Feng, Yanzhong; Chen, Heshu; Li, Fenglan; Du, Zhiliang; Yin, Yulong (17 December 2020). "The Associated Regulatory Mechanisms of Zinc Lactate in Redox Balance and Mitochondrial Function of Intestinal Porcine Epithelial Cells". Oxidative Medicine and Cellular Longevity. 2020: 1–15. doi:10.1155/2020/8815383. PMC 7762675. PMID 33381268.
| Zinc compounds | |||
|---|---|---|---|
| Zinc(I) |
| ||
| Zinc(II) |
| ||