| |

| |
| Names | |
|---|---|
| Other names Zinc diperchlorate, zinc(II) perchlorate | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.033.733 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
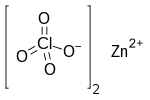
| Chemical formula | Cl 2O 8Zn |
| Molar mass | 261.826 |
| Appearance | colorless solid |
| Density | 2.252 g/cm |
| Melting point | 106 °C (223 °F; 379 K) |
| Boiling point | 210 °C (410 °F; 483 K) |
| Solubility in water | soluble |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Zinc perchlorate is the inorganic compound with the chemical formula Zn(ClO4)2 which forms the hexahydrate.
Synthesis
Zinc perchlorate can be prepared by dissolving zinc oxide or zinc carbonate in perchloric acid:
Chemical properties
The compound decomposes when heated to high temperatures and may explode if heated too strongly.
Like most other perchlorates such as copper perchlorate and lead perchlorate, zinc perchlorate is prone to deliquescence.
Zinc perchlorate can form complexes with ligands such as 8-aminoquinoline, tricarbohydrazide, and tetraphenylethylene tetratriazole.
Physical properties
The compound forms a hexahydrate Zn(ClO
4)
2·6H
2O.
Zinc perchlorate forms a hygroscopic colorless solid, odorless, soluble in water and low-weight alcohols.
Uses
Zinc perchlorate is used as an oxidizing agent and catalyst.
References
- Kumar, Raj; Thilagavathi, Ramasamy; Gulhane, Rajesh; Chakraborti, Asit K. (2 May 2006). "Zinc(II) perchlorate as a new and highly efficient catalyst for formation of aldehyde 1,1-diacetate at room temperature and under solvent-free conditions". Journal of Molecular Catalysis A: Chemical. 250 (1): 226–231. doi:10.1016/j.molcata.2006.01.063. ISSN 1381-1169. Retrieved 14 March 2023.
- Advances in Inorganic Chemistry. Academic Press. 5 December 1984. p. 283. ISBN 978-0-08-057877-4. Retrieved 14 March 2023.
- Lin, Lili; Liu, Xiaohua; Feng, Xiaoming (27 May 2014). "Zinc(II) Perchlorate Hexahydrate". Encyclopedia of Reagents for Organic Synthesis: 1–5. doi:10.1002/047084289X.rn01657. ISBN 9780470842898. Retrieved 14 March 2023.
- "8-aminoquinoline zinc perchlorate metal complex, and preparation method and application thereof". 30 April 2014. Retrieved 14 March 2023.
- "Zinc Perchlorate Hexahydrate". American Elements. Retrieved 14 March 2023.
- "Zinc perchlorate hexahydrate". Sigma Aldrich. Retrieved 14 March 2023.
External links
| Zinc compounds | |||
|---|---|---|---|
| Zinc(I) |
| ||
| Zinc(II) |
| ||

