| Revision as of 02:11, 15 August 2011 editCheMoBot (talk | contribs)Bots141,565 edits Updating {{drugbox}} (no changed fields - added verified revid - updated 'ChemSpiderID_Ref', 'ChEMBL_Ref', 'ChEBI_Ref', 'StdInChI_Ref', 'StdInChIKey_Ref', 'ChEBI_Ref') per Chem/Drugbox validation← Previous edit |
Revision as of 02:12, 3 September 2011 edit undoBogBot (talk | contribs)Bots53,132 edits populated new fields in {{drugbox}} and reordered per bot approval. Report errors and suggestions to User_talk:BogBotNext edit → |
| Line 1: |
Line 1: |
|
{{drugbox |
|
{{Drugbox |
| ⚫ |
| UNII_Ref = {{fdacite|correct|FDA}} |
|
| ⚫ |
| UNII = 4JR41A10VP |
|
|
| verifiedrevid = 443489181 |
|
| verifiedrevid = 443489181 |
|
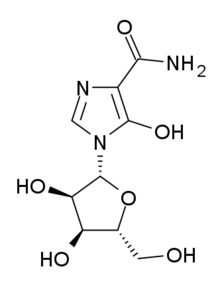
| IUPAC_name = 5-hydroxy-1-β-<small>D</small>-ribofuranosyl-1''H''-imidazole-4-carboxamide |
|
| IUPAC_name = 5-hydroxy-1-β-<small>D</small>-ribofuranosyl-1''H''-imidazole-4-carboxamide |
|
⚫ |
| image = Mizoribine.png |
| ⚫ |
|synonyms = <small>1--5-hydroxyimidazole-4-carboxamide</small> |
|
|
|
|
| ⚫ |
| image = Mizoribine.png |
|
|
|
<!--Clinical data--> |
| ⚫ |
| CAS_number = 50924-49-7 |
|
|
|
| tradename = |
|
| CAS_supplemental = |
|
|
|
| Drugs.com = {{drugs.com|international|mizoribine}} |
| ⚫ |
| ATC_prefix = none |
|
|
| ATC_suffix = |
|
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> |
|
⚫ |
| pregnancy_US = <!-- A / B / C / D / X --> |
| ⚫ |
| ATC_supplemental = |
|
|
⚫ |
| pregnancy_category = |
| ⚫ |
| PubChem = 104762 |
|
|
⚫ |
| legal_AU = <!-- S2, S3, S4, S5, S6, S7, S8, S9 or Unscheduled--> |
|
⚫ |
| legal_CA = <!-- Schedule I, II, III, IV, V, VI, VII, VIII --> |
|
⚫ |
| legal_UK = <!-- GSL, P, POM, CD, or Class A, B, C --> |
|
⚫ |
| legal_US = <!-- OTC / Rx-only / Schedule I, II, III, IV, V --> |
|
⚫ |
| legal_status = Rx-only |
|
⚫ |
| routes_of_administration = Oral |
|
|
|
|
|
<!--Pharmacokinetic data--> |
|
⚫ |
| bioavailability = |
|
⚫ |
| protein_bound = |
|
⚫ |
| metabolism = |
|
⚫ |
| elimination_half-life = |
|
⚫ |
| excretion = |
|
|
|
|
|
<!--Identifiers--> |
|
⚫ |
| CAS_number = 50924-49-7 |
|
⚫ |
| ATC_prefix = none |
|
|
| ATC_suffix = |
|
⚫ |
| ATC_supplemental = |
|
⚫ |
| PubChem = 104762 |
|
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} |
|
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} |
|
| DrugBank = |
|
| DrugBank = |
|
⚫ |
| UNII_Ref = {{fdacite|correct|FDA}} |
|
⚫ |
| UNII = 4JR41A10VP |
|
| KEGG_Ref = {{keggcite|correct|kegg}} |
|
| KEGG_Ref = {{keggcite|correct|kegg}} |
|
| KEGG = D01392 |
|
| KEGG = D01392 |
|
|
|
| ⚫ |
| chemical_formula = |
|
|
|
<!--Chemical data--> |
|
⚫ |
| chemical_formula = |
|
| C=9 | H=13 | N=3 | O=6 |
|
| C=9 | H=13 | N=3 | O=6 |
|
| molecular_weight = 259.21 g/mol |
|
| molecular_weight = 259.21 g/mol |
|
| smiles = C1=NC(=C(N1C2C(C(C(O2)CO)O)O)O)C(=O)N |
|
| smiles = C1=NC(=C(N1C2C(C(C(O2)CO)O)O)O)C(=O)N |
|
⚫ |
| synonyms = <small>1--5-hydroxyimidazole-4-carboxamide</small> |
| ⚫ |
| bioavailability = |
|
| ⚫ |
| protein_bound = |
|
| ⚫ |
| metabolism = |
|
| ⚫ |
| elimination_half-life = |
|
| ⚫ |
| excretion = |
|
|
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> |
|
| ⚫ |
| pregnancy_US = <!-- A / B / C / D / X --> |
|
| ⚫ |
| pregnancy_category= |
|
| ⚫ |
| legal_AU = <!-- S2, S3, S4, S5, S6, S7, S8, S9 or Unscheduled--> |
|
| ⚫ |
| legal_CA = <!-- Schedule I, II, III, IV, V, VI, VII, VIII --> |
|
| ⚫ |
| legal_UK = <!-- GSL, P, POM, CD, or Class A, B, C --> |
|
| ⚫ |
| legal_US = <!-- OTC / Rx-only / Schedule I, II, III, IV, V --> |
|
| ⚫ |
| legal_status = Rx-only |
|
| ⚫ |
| routes_of_administration = Oral |
|
|
}} |
|
}} |
|
|
|
|