| Revision as of 10:09, 7 December 2011 editCheMoBot (talk | contribs)Bots141,565 edits Updating {{drugbox}} (changes to verified fields - added verified revid - updated '') per Chem/Drugbox validation (report errors or bugs)← Previous edit | Revision as of 23:24, 24 December 2011 edit undoCitation bot 1 (talk | contribs)Bots130,044 editsm Add: issue. Tweak: title. You can use this bot yourself. Report bugs here.Next edit → | ||
| Line 62: | Line 62: | ||
| ==Biosynthesis== | ==Biosynthesis== | ||
| Sparteine is a lupin ] containing a tetracyclic bis-quinolizidine ring system derived from three C<sub>5</sub> chains of ], or more specifically, L-lysine.<ref name="NatProdBook">{{cite book |author=Dewick, P.M. |title= Medicinal Natural Products, 3rd. Ed. |publisher=Wiley |pages=311 |year=2009}}</ref> The first intermediate in the biosynthesis is ], the decarboxylation product of lysine catalyzed by the enzyme lysine decarboxylase (LDC).<ref name="Spenser">{{cite journal |doi=10.1139/v88-280 |author=Golebiewski, W.M., Spenser |title=Biosynthesis of the lupine alkaloids. II. Sparteine and lupanine |journal=Can. J. Chem. |volume=66 |pages=1734 |year=1988}}</ref>. Three units of cadaverine are used to form the quinolizidine skeleton. The mechanism of formation has been studied enzymatically, as well as with tracer experiments, but the exact route of synthesis still remains unclear. | Sparteine is a lupin ] containing a tetracyclic bis-quinolizidine ring system derived from three C<sub>5</sub> chains of ], or more specifically, L-lysine.<ref name="NatProdBook">{{cite book |author=Dewick, P.M. |title= Medicinal Natural Products, 3rd. Ed. |publisher=Wiley |pages=311 |year=2009}}</ref> The first intermediate in the biosynthesis is ], the decarboxylation product of lysine catalyzed by the enzyme lysine decarboxylase (LDC).<ref name="Spenser">{{cite journal |doi=10.1139/v88-280 |author=Golebiewski, W.M., Spenser |title=Biosynthesis of the lupine alkaloids. II. Sparteine and lupanine |journal=Can. J. Chem. |volume=66 |pages=1734 |year=1988 |issue=7}}</ref>. Three units of cadaverine are used to form the quinolizidine skeleton. The mechanism of formation has been studied enzymatically, as well as with tracer experiments, but the exact route of synthesis still remains unclear. | ||
| Tracer studies using <sup>13</sup>C-<sup>15</sup>N-doubly labeled cadaverine have shown three units of cadaverine are incorporated into sparteine and two of the C-N bonds from two of the cadaverine units remain intact.<ref name="Rana">{{cite journal |author=Rana, J., Robins, D.J. |title=Quinolizidine alkaloid biosynthesis: incorporation of cadaverine into sparteine |
Tracer studies using <sup>13</sup>C-<sup>15</sup>N-doubly labeled cadaverine have shown three units of cadaverine are incorporated into sparteine and two of the C-N bonds from two of the cadaverine units remain intact.<ref name="Rana">{{cite journal |author=Rana, J., Robins, D.J. |title=Quinolizidine alkaloid biosynthesis: incorporation of cadaverine into sparteine |journal=J. Chem. Soc., Chem. Comm.|volume=22 |pages=1335–6 |year=1983}}</ref> The observations have also been confirmed using <sup>2</sup>H NMR labeling experiments.<ref name="Fraser">{{cite journal |author=Fraser, A.M., Robins, D.J. |journal=J. Chem. Soc., Chem. Comm. |pages=11477 |year=1984}}</ref> | ||
| Enzymatic evidence then showed that the three molecules of cadaverine are transformed to the quinolizidine ring via enzyme bound intermediates, without the generation of any free intermediates. Originally, it was thought that conversion of cadaverine to the corresponding aldehyde, 5-aminopentanal, was catalyzed by the enzyme diamine oxidase.<ref name="SecretLife">{{cite book |author=Aniszewski, T. |title= Alkaloids - Secrets of Life, 1st Ed. |publisher=Elseview |pages=98–101 |year=2007}}</ref> The aldehyde then spontaneously converts to the corresponding Schiff base, Δ<sup>1</sup>-piperideine. Coupling of two molecules occurs between the two tautomers of Δ<sup>1</sup>-piperideine in an aldol-type reaction. The imine is then hydrolyzed to the corresponding aldehyde/amine. The primary amine is then oxidized to an aldehyde followed by formation of the imine to yield the quinolizidine ring.<ref name="SecretLife" /> The breakdown of this mechanism is shown in figure 1; however, the intermediates, as mentioned before, were not isolated. | Enzymatic evidence then showed that the three molecules of cadaverine are transformed to the quinolizidine ring via enzyme bound intermediates, without the generation of any free intermediates. Originally, it was thought that conversion of cadaverine to the corresponding aldehyde, 5-aminopentanal, was catalyzed by the enzyme diamine oxidase.<ref name="SecretLife">{{cite book |author=Aniszewski, T. |title= Alkaloids - Secrets of Life, 1st Ed. |publisher=Elseview |pages=98–101 |year=2007}}</ref> The aldehyde then spontaneously converts to the corresponding Schiff base, Δ<sup>1</sup>-piperideine. Coupling of two molecules occurs between the two tautomers of Δ<sup>1</sup>-piperideine in an aldol-type reaction. The imine is then hydrolyzed to the corresponding aldehyde/amine. The primary amine is then oxidized to an aldehyde followed by formation of the imine to yield the quinolizidine ring.<ref name="SecretLife" /> The breakdown of this mechanism is shown in figure 1; however, the intermediates, as mentioned before, were not isolated. | ||
| Line 70: | Line 70: | ||
| ] | ] | ||
| More recent enzymatic evidence has indicated the presence of 17-oxosparteine synthase (OS), a transaminase enzyme.<ref name="Wink84">{{cite book |author=Wink, M., Hartmann, T. |title=Enzymology of Quinolizidine Alkaloid Biosynthesis; Natural Products Chemistry: Zalewski and Skolik (Eds.) |pages=511–520 |year=1984}}</ref>, <ref name="Wink87">{{cite journal |author=Wink, M. |title=Quinolizidine Alkaloids: Biochemistry, Metabolism, and Function in Plants and Cell Suspension Cultures |
More recent enzymatic evidence has indicated the presence of 17-oxosparteine synthase (OS), a transaminase enzyme.<ref name="Wink84">{{cite book |author=Wink, M., Hartmann, T. |title=Enzymology of Quinolizidine Alkaloid Biosynthesis; Natural Products Chemistry: Zalewski and Skolik (Eds.) |pages=511–520 |year=1984}}</ref>, <ref name="Wink87">{{cite journal |author=Wink, M. |title=Quinolizidine Alkaloids: Biochemistry, Metabolism, and Function in Plants and Cell Suspension Cultures |journal=Plant Medica |pages=509–514 |year=1987}}</ref>, <ref name="Wink79">{{cite journal |doi=10.1016/0014-5793(79)81040-6 |author=Wink, M., Hartmann, T. |title=Cadaverine--pyruvate transamination: the principal step of enzymatic quinolizidine alkaloid biosynthesis in Lupinus polyphyllus cell suspension cultures |journal=FEBS Letters |volume= 101 |issue=2 |pages=343–346 |year=1979 |pmid=446758}}</ref>, <ref name="Perrey">{{cite journal |author=Perrey, R., Wink, M. |journal=Z. Naturfrosh |volume= 43 |pages=363–369 |year=1988}}</ref>, <ref name="Atta">{{cite book |author=Atta-ur-Rahman (Ed.) |title=Natural Products Chemistry |volume= 15 |publisher=Elsevier |pages=537 |year=1995 |isbn=0444426914}}</ref>, <ref name="Roberts">{{cite book |author=Roberts, M., Wink, M. (Eds.) |title=Alkaloids: Biochemistry, Ecology, and Medicinal Applications. |publisher=Plenum Press |pages=112–114 |year=1998}}</ref> The deaminated cadaverine is not released from the enzyme, thus is can be assumed that the enzyme catalyzes the formation of the quinolizidine skeleton in a channeled fashion (Figure 2).<ref name="Perrey" />, <ref name="Atta" />, <ref name="Roberts" /> 7-oxosparteine requires four units of pyruvate as the NH<sub>2</sub> acceptors and produces four molecules of alanine (Figure 3). Both lysine decarboxylase and the quinolizidine skeleton-forming enzyme are localized in chloroplasts.<ref name="Wink80">{{cite journal |author=Wink, M., Hartmann, T. |journal=Z. Naturforsh |volume= 35 |pages=93–97 |year=1980}}</ref> | ||
| ] | ] | ||
Revision as of 23:24, 24 December 2011
Pharmaceutical compound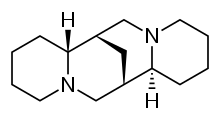 | |
| Clinical data | |
|---|---|
| Other names | (6R,8S,10R,12S)-7,15-diazatetracycloheptadecane |
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.001.808 |
| Chemical and physical data | |
| Formula | C15H26N2 |
| Molar mass | 234.380 g/mol g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (what is this?) (verify) | |
Sparteine is a class 1a antiarrhythmic agent; a sodium channel blocker. It is an alkaloid and can be extracted from scotch broom. It is the predominant alkaloid in Lupinus mutabilis, and is thought to chelate the bivalents calcium and magnesium. It is not FDA approved for human use as an antiarrhythmic agent, and it is not included in the Vaughn Williams classification of antiarrhythmic drugs.
It is also used as a chiral base in organic chemistry, and as a ligand in organic chemical synthesis.
Biosynthesis
Sparteine is a lupin alkaloid containing a tetracyclic bis-quinolizidine ring system derived from three C5 chains of lysine, or more specifically, L-lysine. The first intermediate in the biosynthesis is cadaverine, the decarboxylation product of lysine catalyzed by the enzyme lysine decarboxylase (LDC).. Three units of cadaverine are used to form the quinolizidine skeleton. The mechanism of formation has been studied enzymatically, as well as with tracer experiments, but the exact route of synthesis still remains unclear.
Tracer studies using C-N-doubly labeled cadaverine have shown three units of cadaverine are incorporated into sparteine and two of the C-N bonds from two of the cadaverine units remain intact. The observations have also been confirmed using H NMR labeling experiments.
Enzymatic evidence then showed that the three molecules of cadaverine are transformed to the quinolizidine ring via enzyme bound intermediates, without the generation of any free intermediates. Originally, it was thought that conversion of cadaverine to the corresponding aldehyde, 5-aminopentanal, was catalyzed by the enzyme diamine oxidase. The aldehyde then spontaneously converts to the corresponding Schiff base, Δ-piperideine. Coupling of two molecules occurs between the two tautomers of Δ-piperideine in an aldol-type reaction. The imine is then hydrolyzed to the corresponding aldehyde/amine. The primary amine is then oxidized to an aldehyde followed by formation of the imine to yield the quinolizidine ring. The breakdown of this mechanism is shown in figure 1; however, the intermediates, as mentioned before, were not isolated.

More recent enzymatic evidence has indicated the presence of 17-oxosparteine synthase (OS), a transaminase enzyme., , , , , The deaminated cadaverine is not released from the enzyme, thus is can be assumed that the enzyme catalyzes the formation of the quinolizidine skeleton in a channeled fashion (Figure 2)., , 7-oxosparteine requires four units of pyruvate as the NH2 acceptors and produces four molecules of alanine (Figure 3). Both lysine decarboxylase and the quinolizidine skeleton-forming enzyme are localized in chloroplasts.


See also
References
- Dewick, P.M. (2009). Medicinal Natural Products, 3rd. Ed. Wiley. p. 311.
- Golebiewski, W.M., Spenser (1988). "Biosynthesis of the lupine alkaloids. II. Sparteine and lupanine". Can. J. Chem. 66 (7): 1734. doi:10.1139/v88-280.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Rana, J., Robins, D.J. (1983). "Quinolizidine alkaloid biosynthesis: incorporation of cadaverine into sparteine". J. Chem. Soc., Chem. Comm. 22: 1335–6.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Fraser, A.M., Robins, D.J. (1984). J. Chem. Soc., Chem. Comm.: 11477.
{{cite journal}}: Missing or empty|title=(help)CS1 maint: multiple names: authors list (link) - ^ Aniszewski, T. (2007). Alkaloids - Secrets of Life, 1st Ed. Elseview. pp. 98–101.
- Wink, M., Hartmann, T. (1984). Enzymology of Quinolizidine Alkaloid Biosynthesis; Natural Products Chemistry: Zalewski and Skolik (Eds.). pp. 511–520.
{{cite book}}: CS1 maint: multiple names: authors list (link) - Wink, M. (1987). "Quinolizidine Alkaloids: Biochemistry, Metabolism, and Function in Plants and Cell Suspension Cultures". Plant Medica: 509–514.
- Wink, M., Hartmann, T. (1979). "Cadaverine--pyruvate transamination: the principal step of enzymatic quinolizidine alkaloid biosynthesis in Lupinus polyphyllus cell suspension cultures". FEBS Letters. 101 (2): 343–346. doi:10.1016/0014-5793(79)81040-6. PMID 446758.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Perrey, R., Wink, M. (1988). Z. Naturfrosh. 43: 363–369.
{{cite journal}}: Missing or empty|title=(help)CS1 maint: multiple names: authors list (link) - ^ Atta-ur-Rahman (Ed.) (1995). Natural Products Chemistry. Vol. 15. Elsevier. p. 537. ISBN 0444426914.
- ^ Roberts, M., Wink, M. (Eds.) (1998). Alkaloids: Biochemistry, Ecology, and Medicinal Applications. Plenum Press. pp. 112–114.
{{cite book}}: CS1 maint: multiple names: authors list (link) - Wink, M., Hartmann, T. (1980). Z. Naturforsh. 35: 93–97.
{{cite journal}}: Missing or empty|title=(help)CS1 maint: multiple names: authors list (link)
| Antiarrhythmic agents (C01B) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Channel blockers |
| ||||||||||||
| Receptor agonists and antagonists |
| ||||||||||||
| Ion transporters |
| ||||||||||||
| |||||||||||||