|
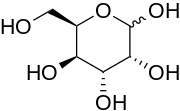
'''Gulose''' is an ] sugar. It is a ] that is very rare in nature, but has been found in ], ] and ].<ref>{{cite journal | author = Swain, M., Brisson, J. R., Sprott, G. D., Cooper, F. P. and Patel, G. B. | title = Identification of β-L-gulose as the sugar moiety of the main polar lipid Thermoplasma acidophilum | journal = Biochim. Biophys. Acta | volume = 1345 | issue = 1 | pages = 56–64 | year = 1997 | pmid = 9084501 | doi=10.1016/s0005-2760(96)00163-4}}</ref> It also exists as a syrup with a sweet taste. It is soluble in water and slightly soluble in ]. Neither the {{sm|d}}- nor {{sm|l}}-forms are fermentable by ]. |
|
'''Gulose''' is an ] sugar. It is a ] that is very rare in nature, but has been found in ], ] and ].<ref>{{cite journal | author = Swain, M., Brisson, J. R., Sprott, G. D., Cooper, F. P. and Patel, G. B. | title = Identification of β-L-gulose as the sugar moiety of the main polar lipid Thermoplasma acidophilum | journal = Biochim. Biophys. Acta | volume = 1345 | issue = 1 | pages = 56–64 | year = 1997 | pmid = 9084501 | doi=10.1016/s0005-2760(96)00163-4}}</ref> It also exists as a syrup with a sweet taste. It is soluble in water and slightly soluble in ]. Neither the {{sm|d}}- nor {{sm|l}}-forms are fermentable by ]. |
|
<small>D</small>-Gulose is a C-3 ] of ] and a C-5 epimer of ].<ref>{{cite journal | author = Zhang, Qingju |display-authors=etal |title=On the Reactivity of Gulose and Guluronic Acid Building Blocks in the Context of Alginate Assembly |journal=European Journal of Organic Chemistry |year=2016 |volume=2016 |issue=14 |pages=2393–2397 |doi=10.1002/ejoc.201600336}}</ref> |
|
<small>D</small>-Gulose is a C-3 ] of ] and a C-5 epimer of ].<ref>{{cite journal | author = Zhang, Qingju |display-authors=etal |title=On the Reactivity of Gulose and Guluronic Acid Building Blocks in the Context of Alginate Assembly |journal=European Journal of Organic Chemistry |year=2016 |volume=2016 |issue=14 |pages=2393–2397 |doi=10.1002/ejoc.201600336}}</ref> |