| Revision as of 08:53, 17 September 2024 edit82.43.53.255 (talk) ChEBI ID added to CHEMBOX identifiers← Previous edit | Latest revision as of 05:56, 22 November 2024 edit undo103.140.83.151 (talk)No edit summaryTags: Mobile edit Mobile web edit | ||
| Line 60: | Line 60: | ||
| }} | }} | ||
| '''Secnidazole''' (trade names '''Flagentyl''', '''Sindose''', '''Secnil''', '''Solosec''') is a ] ]. Effectiveness in the treatment of ] has been reported.<ref>{{cite journal | vauthors = Girginkardeşler N, Coşkun S, Cüneyt Balcioğlu I, Ertan P, Ok UZ | title = Dientamoeba fragilis, a neglected cause of diarrhea, successfully treated with secnidazole | journal = Clinical Microbiology and Infection | volume = 9 | issue = 2 | pages = 110–3 | date = February 2003 | pmid = 12588330 | doi = 10.1046/j.1469-0691.2003.00504.x | doi-access = free }}</ref> It has also been tested against '']''.<ref>{{cite journal | vauthors = De Backer E, Dubreuil L, Brauman M, Acar J, Vaneechoutte M | title = In vitro activity of secnidazole against Atopobium vaginae, an anaerobic pathogen involved in bacterial vaginosis | journal = Clinical Microbiology and Infection | volume = 16 | issue = 5 | pages = 470–2 | date = May 2010 | pmid = 19548924 | doi = 10.1111/j.1469-0691.2009.02852.x | doi-access = free }}</ref> | '''Secnidazole''' (trade names '''Flagentyl''', '''Sindose''', '''Secnil''', '''Solosec''') is a ] ]. Structurally it actually methyl-metronidazole. Effectiveness in the treatment of ] has been reported.<ref>{{cite journal | vauthors = Girginkardeşler N, Coşkun S, Cüneyt Balcioğlu I, Ertan P, Ok UZ | title = Dientamoeba fragilis, a neglected cause of diarrhea, successfully treated with secnidazole | journal = Clinical Microbiology and Infection | volume = 9 | issue = 2 | pages = 110–3 | date = February 2003 | pmid = 12588330 | doi = 10.1046/j.1469-0691.2003.00504.x | doi-access = free }}</ref> It has also been tested against '']''.<ref>{{cite journal | vauthors = De Backer E, Dubreuil L, Brauman M, Acar J, Vaneechoutte M | title = In vitro activity of secnidazole against Atopobium vaginae, an anaerobic pathogen involved in bacterial vaginosis | journal = Clinical Microbiology and Infection | volume = 16 | issue = 5 | pages = 470–2 | date = May 2010 | pmid = 19548924 | doi = 10.1111/j.1469-0691.2009.02852.x | doi-access = free }}</ref> | ||
| In the United States, secnidazole is FDA approved for the treatment of ] and ] in adult women.<ref name="pmid35153156">{{cite journal | vauthors = Muzny CA, Van Gerwen OT | title = Secnidazole for Trichomoniasis in Women and Men | journal = Sex Med Rev | volume = 10 | issue = 2 | pages = 255–262 | date = April 2022 | pmid = 35153156 | doi = 10.1016/j.sxmr.2021.12.004 | s2cid = 246755406 | doi-access = free | pmc = 11019772 }}</ref> | In the United States, secnidazole is FDA approved for the treatment of ] and ] in adult women.<ref name="pmid35153156">{{cite journal | vauthors = Muzny CA, Van Gerwen OT | title = Secnidazole for Trichomoniasis in Women and Men | journal = Sex Med Rev | volume = 10 | issue = 2 | pages = 255–262 | date = April 2022 | pmid = 35153156 | doi = 10.1016/j.sxmr.2021.12.004 | s2cid = 246755406 | doi-access = free | pmc = 11019772 }}</ref> | ||
Latest revision as of 05:56, 22 November 2024
Chemical compoundPharmaceutical compound
 | |
| Clinical data | |
|---|---|
| Trade names | Solosec |
| Other names | PM 185184, RP 14539 |
| AHFS/Drugs.com | International Drug Names |
| License data |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.020.123 |
| Chemical and physical data | |
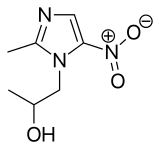
| Formula | C7H11N3O3 |
| Molar mass | 185.183 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (what is this?) (verify) | |
Secnidazole (trade names Flagentyl, Sindose, Secnil, Solosec) is a nitroimidazole anti-infective. Structurally it actually methyl-metronidazole. Effectiveness in the treatment of dientamoebiasis has been reported. It has also been tested against Atopobium vaginae.
In the United States, secnidazole is FDA approved for the treatment of bacterial vaginosis and trichomoniasis in adult women.
References
- Girginkardeşler N, Coşkun S, Cüneyt Balcioğlu I, Ertan P, Ok UZ (February 2003). "Dientamoeba fragilis, a neglected cause of diarrhea, successfully treated with secnidazole". Clinical Microbiology and Infection. 9 (2): 110–3. doi:10.1046/j.1469-0691.2003.00504.x. PMID 12588330.
- De Backer E, Dubreuil L, Brauman M, Acar J, Vaneechoutte M (May 2010). "In vitro activity of secnidazole against Atopobium vaginae, an anaerobic pathogen involved in bacterial vaginosis". Clinical Microbiology and Infection. 16 (5): 470–2. doi:10.1111/j.1469-0691.2009.02852.x. PMID 19548924.
- Muzny CA, Van Gerwen OT (April 2022). "Secnidazole for Trichomoniasis in Women and Men". Sex Med Rev. 10 (2): 255–262. doi:10.1016/j.sxmr.2021.12.004. PMC 11019772. PMID 35153156. S2CID 246755406.
Further reading
- Gillis JC, Wiseman LR (April 1996). "Secnidazole. A review of its antimicrobial activity, pharmacokinetic properties and therapeutic use in the management of protozoal infections and bacterial vaginosis". Drugs. 51 (4): 621–38. doi:10.2165/00003495-199651040-00007. PMID 8706597. S2CID 195692679.
| Antiparasitics – antiprotozoal agents – agents against amoebozoa/amebicide (P01) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Entamoeba |
| ||||||||||||||||
| Acanthamoeba | |||||||||||||||||
| |||||||||||||||||
This antiinfective drug article is a stub. You can help Misplaced Pages by expanding it. |