| Revision as of 21:05, 2 December 2010 editRjwilmsi (talk | contribs)Extended confirmed users, Pending changes reviewers, Rollbackers932,080 editsm formatting journal PMID cites using AWB (7419)← Previous edit | Revision as of 21:07, 2 December 2010 edit undoCitation bot (talk | contribs)Bots5,433,741 editsm Citations: added: doi, last4, first4, last5, first5, last6, first6. Tweaked: last1, first1, last2, first2, last3, first3, year, title, journal, volume, issue, pages. RjwilmsiNext edit → | ||
| Line 27: | Line 27: | ||
| }} | }} | ||
| '''Evoxine''' ('''Haploperine''') is an ] with ] and ] effects. It is found naturally in a variety of Australian and African plants including '']''<ref>{{cite journal | last1 = Eastwood | first1 = FW | last2 = Hughes | first2 = GK | last3 = Ritchie | first3 = E. | year = 1954 | title = Alkaloids of the Australian Rutaceae: Evodia xanthoxyloides F.Muell. IV. The structures of Evoxine and Evoxoidine | url = | journal = Australian Journal of Chemistry | volume = 7 | issue = 1| pages = 87–98 }}</ref> and '']''.<ref>{{cite journal | pmid = 17174364 }}</ref> | '''Evoxine''' ('''Haploperine''') is an ] with ] and ] effects. It is found naturally in a variety of Australian and African plants including '']''<ref>{{cite journal | doi = 10.1071/CH9540087 | last1 = Eastwood | first1 = FW | last2 = Hughes | first2 = GK | last3 = Ritchie | first3 = E. | year = 1954 | title = Alkaloids of the Australian Rutaceae: Evodia xanthoxyloides F.Muell. IV. The structures of Evoxine and Evoxoidine | url = | journal = Australian Journal of Chemistry | volume = 7 | issue = 1| pages = 87–98 }}</ref> and '']''.<ref>{{cite journal | last1 = Waffo | first1 = AF | last2 = Coombes | first2 = PH | last3 = Crouch | first3 = NR | last4 = Mulholland | first4 = DA | last5 = El Amin | first5 = SM | last6 = Smith | first6 = PJ | title = Acridone and furoquinoline alkaloids from Teclea gerrardii (Rutaceae: Toddalioideae) of southern Africa. | journal = Phytochemistry | volume = 68 | issue = 5 | pages = 663–7 | year = 2007 | pmid = 17174364 | doi = 10.1016/j.phytochem.2006.10.011 }}</ref> | ||
| ==References== | ==References== | ||
Revision as of 21:07, 2 December 2010
Pharmaceutical compound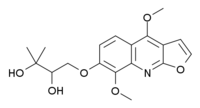 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C11H12O2 |
| Molar mass | 347.362 g/mol g·mol |
| 3D model (JSmol) | |
SMILES
| |
Evoxine (Haploperine) is an alkaloid with hypnotic and sedative effects. It is found naturally in a variety of Australian and African plants including Evodia xanthoxyloides and Teclea gerrardii.
References
- Eastwood, FW; Hughes, GK; Ritchie, E. (1954). "Alkaloids of the Australian Rutaceae: Evodia xanthoxyloides F.Muell. IV. The structures of Evoxine and Evoxoidine". Australian Journal of Chemistry. 7 (1): 87–98. doi:10.1071/CH9540087.
- Waffo, AF; Coombes, PH; Crouch, NR; Mulholland, DA; El Amin, SM; Smith, PJ (2007). "Acridone and furoquinoline alkaloids from Teclea gerrardii (Rutaceae: Toddalioideae) of southern Africa". Phytochemistry. 68 (5): 663–7. doi:10.1016/j.phytochem.2006.10.011. PMID 17174364.
This sedative-related article is a stub. You can help Misplaced Pages by expanding it. |