This is an old revision of this page, as edited by Beetstra (talk | contribs) at 13:11, 24 November 2011 (Script assisted update of identifiers for the Chem/Drugbox validation project (updated: 'ChEMBL').). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 13:11, 24 November 2011 by Beetstra (talk | contribs) (Script assisted update of identifiers for the Chem/Drugbox validation project (updated: 'ChEMBL').)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff) Pharmaceutical compound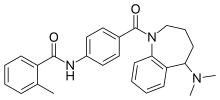 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C27H29N3O2 |
| Molar mass | 427.53 g/mol g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Mozavaptan (INN) is a vasopressin receptor antagonist marketed by Otsuka. In Japan, it was approved in October 2006 for hyponatremia (low blood sodium levels) caused by syndrome of inappropriate antidiuretic hormone (SIADH) due to ADH producing tumors.
References
- H. Spreitzer (November 20, 2006). "Neue Wirkstoffe - Conivaptan". Österreichische Apothekerzeitung (in German) (24/2006).
- Prous Science: Molecule of the Month November 2006
| Diuretics (C03) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sulfonamides (and etacrynic acid) |
| ||||||||
| Potassium-sparing (at CD) |
| ||||||||
| Osmotic diuretics (PT, DL) | |||||||||
| Vasopressin receptor inhibitors (DCT and CD) | |||||||||
| Other | |||||||||
| Combination products | |||||||||
| |||||||||
Template:Neuropeptide agonists and antagonists
This antihypertensive-related article is a stub. You can help Misplaced Pages by expanding it. |