This is an old revision of this page, as edited by 192.122.131.20 (talk) at 02:53, 1 February 2012 (→Nomenclature: changed broken link). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 02:53, 1 February 2012 by 192.122.131.20 (talk) (→Nomenclature: changed broken link)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)
| |
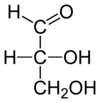
| |
| Names | |
|---|---|
| IUPAC name 2,3-Dihydroxypropanal | |
| Other names
Glyceraldehyde Glyceric aldehyde | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.000.264 |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C3H6O3 |
| Molar mass | 90.078 g·mol |
| Density | 1.455 g/cm³ |
| Melting point | 145 °C (293 °F; 418 K) |
| Boiling point | 140−150 °C at 0.8 mmHg |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Glyceraldehyde is a triose monosaccharide with chemical formula C3H6O3. It is the simplest of all common aldoses. It is a sweet, colorless, crystalline solid that is an intermediate compound in carbohydrate metabolism. The word comes from combining glycerine and aldehyde, as glyceraldehyde is merely glycerine with one hydroxymethylene group changed to an aldehyde.
Structure
Glyceraldehyde has chiral center and therefore exists as two different enantiomers with opposite optical rotation:
- R from Latin rectus meaning "right", or
- S from Latin sinister meaning "left"
| d-glyceraldehyde (R)-glyceraldehyde (+)-glyceraldehyde |
l-glyceraldehyde (S)-glyceraldehyde (−)-glyceraldehyde | |
| Fischer projection | 
|

|
| Skeletal formula | 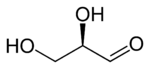
|

|
| Ball-and-stick model | 
|
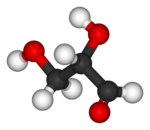
|
While the optical rotation of glyceraldehyde is (+) for R and (−) for S, this is not true for all monosaccharides. The stereochemical rotation can only be determined by the chemical structure, whereas the optical rotation can only be determined empirically (by experiment).
It was by a lucky guess that the molecular d- geometry was assigned to (+)-glyceraldehyde in the late 19th century, as confirmed by X-ray crystallography in 1951.
Nomenclature
In the d/l system, glyceraldehyde is used as the configurational standard for carbohydrates. Monosaccharides with a conformation identical to (R)-glyceraldehyde at the last stereocentre, for example C5 in glucose, are assigned the stereo-descriptor d-. Those similar to (S)-glyceraldehyde are assigned an l-.
Synthesis and biochemical role
Glyceraldehyde can be prepared, along with dihydroxyacetone, by the mild oxidation of glycerol, for example with hydrogen peroxide and a ferrous salt as catalyst. Dihydroxyacetone, the simplest ketose, is an isomer of glyceraldehyde. Interconversion of the phosphates of these two compounds, catalyzed by the enzyme triosephosphate isomerase, is an important intermediate step in glycolysis.
See also
References
- Merck Index, 11th Edition, 4376
| Types of carbohydrates | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| General | |||||||||||||||
| Geometry | |||||||||||||||
| Monosaccharides |
| ||||||||||||||
| Multiple |
| ||||||||||||||
| Fructose and galactose metabolic intermediates | |||||||
|---|---|---|---|---|---|---|---|
| Fructose | |||||||
| Galactose | |||||||
| Mannose | |||||||