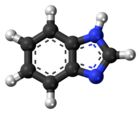
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name 1H-1,3-Benzimidazole | |||
| Other names 1H-Benzoimidazole | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| Beilstein Reference | 109682 | ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.000.075 | ||
| EC Number |
| ||
| Gmelin Reference | 3106 | ||
| KEGG | |||
| PubChem CID | |||
| UNII | |||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | C7H6N2 | ||
| Molar mass | 118.139 g·mol | ||
| Melting point | 170 to 172 °C (338 to 342 °F; 443 to 445 K) | ||
| Acidity (pKa) | 12.8 (for benzimidazole) and 5.6 (for the conjugate acid) | ||
| Hazards | |||
| GHS labelling: | |||
| Pictograms | 
| ||
| Signal word | Warning | ||
| Hazard statements | H302, H315, H319, H335 | ||
| Precautionary statements | P261, P264, P270, P271, P280, P301+P312, P302+P352, P304+P340, P305+P351+P338, P312, P321, P330, P332+P313, P337+P313, P362, P403+P233, P405, P501 | ||
| Safety data sheet (SDS) | External MSDS | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Benzimidazole is a heterocyclic aromatic organic compound. This bicyclic compound may be viewed as fused rings of the aromatic compounds benzene and imidazole. It is a white solid that appears in form of tabular crystals.
Preparation
Benzimidazole was discovered during research on vitamin B12. The benzimidazole nucleus was found to be a stable platform on which drugs could be developed. Benzimidazole is produced by condensation of o-phenylenediamine with formic acid, or the equivalent trimethyl orthoformate:
- C6H4(NH2)2 + HC(OCH3)3 → C6H4N(NH)CH + 3 CH3OH
2-Substituted derivatives are obtained when the condensation is conducted with aldehydes in place of formic acid, followed by oxidation.
Reactions
Benzimidazole is a base:
- C6H4N(NH)CH + H →
It can also be deprotonated with stronger bases:
- C6H4N(NH)CH + LiH → Li + H2
The imine can be alkylated and also serves as a ligand in coordination chemistry. The most prominent benzimidazole complex features N-ribosyl-dimethylbenzimidazole, as found in vitamin B12.
N,N'-Dialkylbenzimidazolium salts are precursors to certain N-heterocyclic carbenes.
Applications
Benzimidazole derivatives are among the most frequently used ring systems for small molecule drugs listed by the United States Food and Drug Administration. Many pharmaceutical agents belong to the benzimidazole class of compounds. For example:
- Angiotensin II receptor blockers such as azilsartan, candesartan, and telmisartan.
- Anthelmintic agents such as albendazole, ciclobendazole, fenbendazole, flubendazole, mebendazole, oxfendazole, oxibendazole, triclabendazole, and thiabendazole. These drugs work by binding tubulin, a vital part of the cytoskeleton and mitotic spindle. Benzimidazoles are selectively toxic towards parasitic nematodes, selectively binding and depolymerising their tubulins.
- Antihistamines such as astemizole, bilastine, clemizole, emedastine, mizolastine, and oxatomide.
- Benzimidazole fungicides such as benomyl, carbendazim, fuberidazole, and thiabendazole. These drugs selectively bind to and depolymerise fungal tubulin.
- Benzimidazole opioids such as bezitramide, brorphine, clonitazene, etodesnitazene, etonitazene, etonitazepipne, etonitazepyne, isotonitazene, metodesnitazene, and metonitazene.
- Proton-pump inhibitors such as dexlansoprazole, esomeprazole, ilaprazole, lansoprazole, omeprazole, pantoprazole, rabeprazole, and tenatoprazole.
- Typical antipsychotics such as benperidol, clopimozide, droperidol, neflumozide, and oxiperomide, and pimozide.
- Other notable pharmaceutical agents which contain a benzimidazole group include abemaciclib, bendamustine, dabigatran, daridorexant, and glasdegib.
In printed circuit board manufacturing, benzimidazole can be used as an organic solderability preservative.
Several dyes are derived from benzimidazoles.
See also
- Benzimidazoline
- Polybenzimidazole, a high performance fiber
References
- Walba, Harold; Isensee, Robert W. (1961). "Acidity Constants of Some Arylimidazoles and Their Cations". The Journal of Organic Chemistry. 26 (8): 2789–2791. doi:10.1021/jo01066a039.
- "Benzimidazole | CAMEO Chemicals | NOAA". cameochemicals.noaa.gov. Retrieved 2023-01-11.
- Bennet-Jenkins, E.; Bryant, C. (1996). "Novel sources of anthelmintics". International Journal for Parasitology. 26 (8–9): 937–947. doi:10.1016/s0020-7519(96)80068-3. ISSN 0020-7519. PMID 8923141.
- E. C. Wagner, W. H. Millett (1939). "Benzimidazole". Organic Syntheses. 19: 12. doi:10.15227/orgsyn.019.0012.
- Smiley, Robert A. (2000), "Phenylene- and Toluenediamines", Ullmann's Encyclopedia of Industrial Chemistry, doi:10.1002/14356007.a19_405, ISBN 978-3-527-30385-4
- H. A. Barker; R. D. Smyth; H. Weissbach; J. I. Toohey; J. N. Ladd & B. E. Volcani (February 1, 1960). "Isolation and Properties of Crystalline Cobamide Coenzymes Containing Benzimidazole or 5,6-Dimethylbenzimidazole". Journal of Biological Chemistry. 235 (2): 480–488. doi:10.1016/S0021-9258(18)69550-X. PMID 13796809.
- R. Jackstell; A. Frisch; M. Beller; D. Rottger; M. Malaun; B. Bildstein (2002). "Efficient telomerization of 1,3-butadiene with alcohols in the presence of in situ generated palladium(0)carbene complexes". Journal of Molecular Catalysis A: Chemical. 185 (1–2): 105–112. doi:10.1016/S1381-1169(02)00068-7.
- H. V. Huynh; J. H. H. Ho; T. C. Neo; L. L. Koh (2005). "Solvent-controlled selective synthesis of a trans-configured benzimidazoline-2-ylidene palladium(II) complex and investigations of its Heck-type catalytic activity". Journal of Organometallic Chemistry. 690 (16): 3854–3860. doi:10.1016/j.jorganchem.2005.04.053.
- Taylor, R. D.; MacCoss, M.; Lawson, A. D. G. J Med Chem 2014, 57, 5845.>
- ^ Wang, C. C. (January 1984). "Parasite enzymes as potential targets for antiparasitic chemotherapy". Journal of Medicinal Chemistry. 27 (1): 1–9. doi:10.1021/jm00367a001. ISSN 0022-2623. PMID 6317859.
- Berneth, Horst (2008), "Methine Dyes and Pigments", Ullmann's Encyclopedia of Industrial Chemistry, doi:10.1002/14356007.a16_487.pub2, ISBN 978-3-527-30385-4
Further reading
- Grimmett, M. R. (1997). Imidazole and benzimidazole synthesis. Boston: Academic Press. ISBN 0-12-303190-7.
| Simple aromatic rings | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 ring |
| ||||||||||||
| 2 rings |
| ||||||||||||
| Antiparasitics – Anthelmintics (P02) and endectocides (QP54) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antiplatyhelmintic agents |
| ||||||||||||||
| Antinematodal agents (including macrofilaricides) |
| ||||||||||||||
| |||||||||||||||
| Antifungals (D01 and J02) | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wall/ membrane |
| ||||||||||||||||||||||
| Intracellular |
| ||||||||||||||||||||||
| Others |
| ||||||||||||||||||||||
| |||||||||||||||||||||||