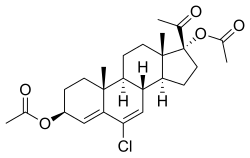
 | |
| Clinical data | |
|---|---|
| Other names | Chlormadinol acetate; AY-11440; 3β,17α-Diacetoxy-6-chloropregna-4,6-diene-20-one |
| Drug class | Progestogen; Progestogen ester |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C25H33ClO5 |
| Molar mass | 448.98 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Clogestone acetate (USANTooltip United States Adopted Name) (developmental code name AY-11440), also known as chlormadinol acetate or as 3β,17α-diacetoxy-6-chloropregna-4,6-diene-20-one, is a steroidal progestin which was investigated as a progestin-only contraceptive and postcoital contraceptive but was never marketed. It is the diacetate ester of clogestone, which, similarly was never marketed. Clogestone acetate produces chlormadinone acetate as an active metabolite.
See also
References
- Litwack G (2 December 2012). Biochemical Actions of Hormones. Elsevier. pp. 323–. ISBN 978-0-323-15189-4.
- ^ Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 297–. ISBN 978-1-4757-2085-3.
- Hawkins DR, Elder MG (22 October 2013). Human Fertility Control: Theory and Practice. Elsevier Science. pp. 3–. ISBN 978-1-4831-6361-1.
- Harper MJ (8 March 2013). "Contraception — retrospect and prospect". Progress in Drug Research, vol. 21. Progress in Drug Research / Fortschritte der Arzneimittelforschung / Progrès des rechersches pharmaceutiques. Vol. 21. Birkhäuser. pp. 293–407. doi:10.1007/978-3-0348-7098-6_4. ISBN 978-3-0348-7098-6. PMID 339271.
- Hinselmann M, Jürgensen O, Hasselblatt I, Otten U, Prinz W, Taubert HD (August 1970). "". Archiv Fur Gynakologie (in German). 209 (2): 136–148. doi:10.1007/BF00668180. PMID 5537778.
- Stern MD, Givner ML (April 1975). "Measurement of serum clogestone acetate (AY-11,440) by a radioreceptor assay: a practical approach to the quantitative determination of synthetic progestins". The Journal of Clinical Endocrinology and Metabolism. 40 (4): 728–731. doi:10.1210/jcem-40-4-728. PMID 1127082.
This drug article relating to the genito-urinary system is a stub. You can help Misplaced Pages by expanding it. |
This article about a steroid is a stub. You can help Misplaced Pages by expanding it. |