Chemical compound Pharmaceutical compound
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C20H23ClO2 |
| Molar mass | 330.85 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Ethynerone (INN, USAN), also known as 17α-(2-chloroethynyl)estra-4,9-dien-17β-ol-3-one, is a steroidal progestin of the 19-nortestosterone group that was first reported in 1961 but was never marketed. Under the developmental code name MK-665, it was studied in combination with mestranol as an oral contraceptive. Development of the drug was discontinued due to concerns surrounding toxicity findings in dogs. It is a chloroethynylated derivative of norethisterone.
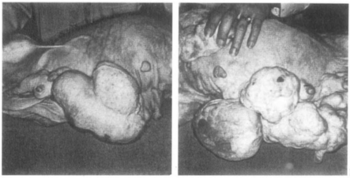
In 1966, during its clinical development, ethynerone was found to produce mammary gland tumors in dogs treated with it at very high doses for prolonged periods of time. Subsequent investigation found that 17α-hydroxyprogesterone derivatives included anagestone acetate, chlormadinone acetate, medroxyprogesterone acetate, and megestrol acetate produced similar mammary gland tumors, and that their ability to do so correlated directly with their progestogenic actions. In contrast, the non-halogenated 19-nortestosterone derivatives norgestrel, norethisterone, noretynodrel, and etynodiol diacetate, which are much less potent as progestogens, did not produce such effects at the dosages tested. Clinical development of ethynerone was discontinued, and many of the 17α-hydroxyprogesterone derivatives were withdrawn for the indication of hormonal contraception. Research later on revealed species differences between dogs and humans and established that there is no similar risk in humans.
Synthesis

See also
References
- Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 521–. ISBN 978-1-4757-2085-3.
- ^ Runnebaum BC, Rabe T, Kiesel L (6 December 2012). Female Contraception: Update and Trends. Springer Science & Business Media. pp. 134–135. ISBN 978-3-642-73790-9.
- Diczfalusy E, et al. (World Health Organization) (1974). Acta Endocrinologica: Supplementum. Ejnar Munksgaard. p. 261. ISBN 9788774940968.
- Geil RG, Lamar JK (September 1977). "FDA studies of estrogen, progestogens, and estrogen/progestogen combinations in the dog and monkey". Journal of Toxicology and Environmental Health. 3 (1–2): 179–93. Bibcode:1977JTEH....3..179G. doi:10.1080/15287397709529557. PMID 411941.
- Jacobs AC, Hatfield KP (March 2013). "History of chronic toxicity and animal carcinogenicity studies for pharmaceuticals". Veterinary Pathology. 50 (2): 324–33. doi:10.1177/0300985812450727. PMID 22700852. S2CID 22367595.
- ^ Lingeman CH (6 December 2012). Carcinogenic Hormones. Springer Science & Business Media. pp. 149–. ISBN 978-3-642-81267-5.
- ^ James VH, Pasqualini JR (22 October 2013). Hormonal Steroids: Proceedings of the Fifth International Congress on Hormonal Steroids. Elsevier Science. pp. 7–8. ISBN 978-1-4831-5895-2.
- p165 Lednicer Mitscher book 1 and p146 (2)
This drug article relating to the genito-urinary system is a stub. You can help Misplaced Pages by expanding it. |
This article about a steroid is a stub. You can help Misplaced Pages by expanding it. |