 | |
| Clinical data | |
|---|---|
| Other names | ORG-30659; Exogestin; Letogestin; 11-Methylene-δ-norethisterone; 17α-Ethynyl-11-methylene-19-nor-δ-testosterone |
| Routes of administration | By mouth |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C21H24O2 |
| Molar mass | 308.421 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
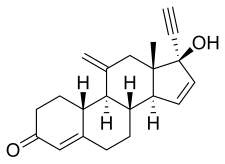
Tosagestin (INNTooltip International Nonproprietary Name, USANTooltip United States Adopted Name) (developmental code name ORG-30659), also known as 11-methylene-δ-norethisterone or 17α-ethynyl-11-methylene-19-nor-δ-testosterone, is a progestin of the 19-nortestosterone group which was under development by Organon in the United States and Europe as a hormonal contraceptive (in combination with ethinylestradiol) and for the treatment of menopausal symptoms but was never marketed.
See also
References
- Negwer M, Scharnow HG (2001). Organic-chemical drugs and their synonyms: (an international survey). Wiley-VCH. p. 2104. ISBN 978-3-527-30247-5.
- World Health Organization (2013), The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances (PDF), p. 95, archived from the original (PDF) on August 8, 2016
- "Tosagestin", AdisInsight, Springer Nature Switzerland AG, retrieved 12 July 2016
- Verhoeven CH, Krebbers SF, Wagenaars GN, Booy CJ, Groothuis GM, Olinga P, Vos RM (November 1998). "In vitro and in vivo metabolism of the progestagen Org 30659 in several species". Drug Metabolism and Disposition. 26 (11): 1102–1112. PMID 9806953.
- de Visser SJ, Uchida N, van Vliet-Daskalopoulou E, Fukazawa I, van Doorn MB, van den Heuvel MW, et al. (September 2003). "Pharmacokinetic differences between Caucasian and Japanese subjects after single and multiple doses of a potential combined oral contraceptive (Org 30659 and EE)". Contraception. 68 (3): 195–202. doi:10.1016/S0010-7824(03)00140-9. PMID 14561540.
- Obruca A, Korver T, Huber J, Killick SR, Landgren B, Struijs MJ (July 2001). "Ovarian function during and after treatment with the new progestagen Org 30659". Fertility and Sterility. 76 (1): 108–115. doi:10.1016/S0015-0282(01)01824-6. PMID 11438328.
This drug article relating to the genito-urinary system is a stub. You can help Misplaced Pages by expanding it. |
This article about a steroid is a stub. You can help Misplaced Pages by expanding it. |