 | |
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C18H23FN4O5 |
| Molar mass | 394.403 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (what is this?) (verify) | |
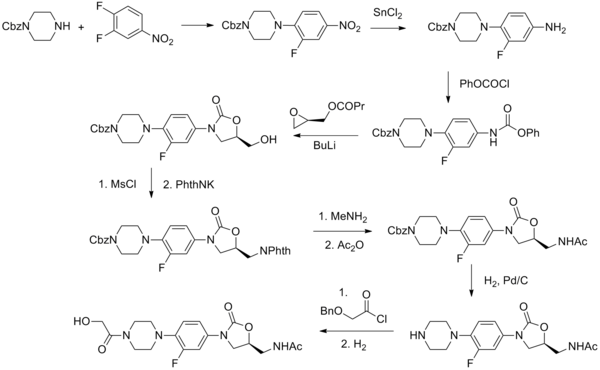
Eperezolid is an oxazolidinone antibiotic.
Synthesis
See also
References
- Brickner SJ, Hutchinson DK, Barbachyn MR, Manninen PR, Ulanowicz DA, Garmon SA, et al. (February 1996). "Synthesis and antibacterial activity of U-100592 and U-100766, two oxazolidinone antibacterial agents for the potential treatment of multidrug-resistant gram-positive bacterial infections". Journal of Medicinal Chemistry. 39 (3): 673–9. doi:10.1021/jm9509556. PMID 8576909.
| Antibacterials that inhibit protein synthesis (J01A, J01B, J01F, J01G, QJ01XQ) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 30S |
| ||||||||||||||
| 50S | |||||||||||||||
| |||||||||||||||
This systemic antibiotic-related article is a stub. You can help Misplaced Pages by expanding it. |