| |
| |
| Names | |
|---|---|
| IUPAC name iron(3+) hexacyanide | |
| Systematic IUPAC name hexacyanidoferrate(III) | |
| Other names ferric hexacyanide; hexacyanidoferrate(3−); hexacyanoferrate(III) | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| KEGG | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | |
| Related compounds | |
| Other cations | Hexacyanonickelate(III) |
| Related compounds | Ferrocyanide |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
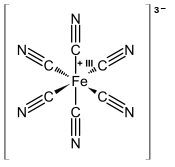
Ferricyanide is the name of the anion . It is also called hexacyanoferrate(III) and in rare, but systematic nomenclature, hexacyanidoferrate(III). The most common salt of this anion is potassium ferricyanide, a red crystalline material that is used as an oxidant in organic chemistry.
Properties
consists of a Fe center bound in octahedral geometry to six cyanide ligands. The complex has Oh symmetry. The iron is low spin and easily reduced to the related ferrocyanide ion , which is a ferrous (Fe) derivative. This redox couple is reversible and entails no making or breaking of Fe–C bonds:
- + e ⇌
This redox couple is a standard in electrochemistry.
Compared to main group cyanides like potassium cyanide, ferricyanides are much less toxic because of the strong bond between the cyanide ion (CN) and the Fe. They do react with mineral acids, however, to release highly toxic hydrogen cyanide gas.
Uses
Treatment of ferricyanide with iron(II) salts affords the brilliant, long-lasting pigment Prussian blue, the traditional color of blueprints.
See also
References
- Gail, E.; Gos, S.; Kulzer, R.; Lorösch, J.; Rubo, A.; Sauer, M.; Kellens, R.; Reddy, J.; Steier, N.; Hasenpusch, W. (October 2011). "Cyano Compounds, Inorganic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a08_159.pub3. ISBN 978-3527306732.
| Salts and covalent derivatives of the cyanide ion | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||