 Skeletal formula of iron(II) citrate | |
 Powdered Iron(II) citrate hydrate | |
| Names | |
|---|---|
| IUPAC name Iron(II) hydrogen 2-hydroxy-1,2,3-tricarboxylpropane | |
| Other names Iron(II) citrate, Ferrous citrate, Iron citrate | |
| Identifiers | |
| CAS Number |
|
| 3D model (JSmol) |
|
| ChemSpider |
|
| ECHA InfoCard | 100.041.463 |
| EC Number |
|
| PubChem CID |
|
| UNII |
|
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | FeC6H6O7 |
| Molar mass | 245.95644 g/mol |
| Appearance | slightly gray-green powder or white crystals unstable |
| Density | 1.91 g/cm |
| Melting point | decomposes |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Ferrous citrate, also known as iron(II) citrate or iron(2+) citrate, describes coordination complexes containing citrate anions with Fe formed in aqueous solution. Although a number of complexes are possible (or even likely), only one complex has been crystallized. That complex is the coordination polymer with the formula {}22H2O, where C6H5O7 is HOC(CH2CO2)2(CO2, i.e., the triple conjugate base of citric acid wherein the three carboxylic acid groups are ionized. Ferrous citrates are all paramagnetic, reflecting the weak crystal field of the carboxylate ligands.
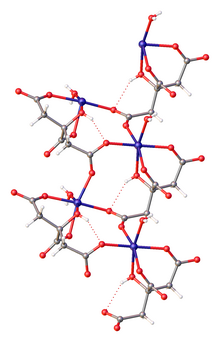
Ferrous citrates are produced by treating disodium citrate Na
2C
6H
6O
7 with sources of iron(II) aquo complexes, such as iron(II) sulfate. Ferrous citrates are all highly unstable in air, converting to ferric citrates.
It is a nutrient supplement approved by the FDA.
See also
References
- Food Chemicals Codex. United States Pharmacopeial Convention. 2010. p. 396. ISBN 978-1-889788-86-9.
- ^ Perry, Dale L.; Phillips, Sidney L., eds. (1995). Handbook of Inorganic Compounds. Boca Raton, Florida: CRC Press. p. 167. ISBN 0-8493-8671-3.
- ^ Strouse, Jane; Layten, Steven W.; Strouse, Charles E. (1977). "Structural Studies of transition metal complexes of triionized and tetraionized citrate. Models for the coordination of the citrate ion to transition metal ions in solution and at the active site of aconitase". Journal of the American Chemical Society. 99 (2): 562–572. doi:10.1021/ja00444a041. PMID 830693.
- Pierre, J. L.; Gautier-Luneau, I. (2000). "Iron and Citric Acid: A Fuzzy Chemistry of Ubiquitous Biological Relevance". Biometals. 13 (1): 91–96. doi:10.1023/A:1009225701332. PMID 10831230. S2CID 2301450.
- "CFR - Code of Federal Regulations Title 21". www.fda.gov. U.S. Food and Drug Administration. 2013-06-01. Retrieved 2014-08-02.
- PubChem. "Iron(II) citrate". pubchem.ncbi.nlm.nih.gov. Retrieved 2022-08-22.
- "Substances Added to Food (formerly EAFUS)". www.cfsanappsexternal.fda.gov. Retrieved 2022-08-22.
| Iron compounds | |||
|---|---|---|---|
| Fe(−II) | |||
| Fe(0) | |||
| Fe(I) |
| ||
| Fe(0,II) | |||
| Fe(II) |
| ||
| Fe(0,III) | |||
| Fe(II,III) | |||
| Fe(III) |
| ||
| Fe(IV) | |||
| Fe(VI) | |||
| Purported | |||
| sort | |||
This article about an organic compound is a stub. You can help Misplaced Pages by expanding it. |