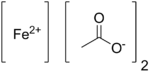
| |
| Names | |
|---|---|
| IUPAC name Iron(II) acetate | |
| Other names Ferrous acetate | |
| Identifiers | |
| CAS Number |
|
| 3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.019.492 |
| PubChem CID | |
| RTECS number |
|
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C4H6FeO4 |
| Molar mass | 173.933 g·mol |
| Appearance | White crystals (anhydrous) Light green crystals (tetrahydrate) |
| Odor | Odorless |
| Density | 1.734 g/cm (−73 °C) |
| Melting point | 190–200 °C (374–392 °F; 463–473 K) decomposes |
| Solubility in water | Soluble |
| Structure | |
| Crystal structure | Orthorhombic, oP75 (200 K) |
| Space group | Pbcn, No. 60 (200 K) |
| Point group | 2/m 2/m 2/m (200 K) |
| Lattice constant | a = 18.1715(4) Å, b = 22.1453(5) Å, c = 8.2781(2) Å (200 K)α = 90°, β = 90°, γ = 90° |
| Hazards | |
| GHS labelling: | |
| Pictograms | 
|
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P261, P305+P351+P338 |
| NFPA 704 (fire diamond) |
 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Iron(II) acetate describes compounds with formula Fe(CH3CO2)2·(H2O)x where x can be 0 (anhydrous) or 4 (tetrahydrate). The anhydrous compound is a white solid, although impure samples can be slightly colored. The tetrahydrate is light green solid that is highly soluble in water.
Preparation and structure

Iron powder reacts with acetic acid to give the ferrous acetate, with evolution of hydrogen gas:
- Fe + 2 CH3CO2H → Fe(CH3CO2)2 + H2
Reaction of scrap iron with acetic acid affords a brown mixture of various iron(II) and iron(III) acetates that are used in dyeing.
It can also be made from the insoluble, olive green, Iron(II) carbonate.
It adopts a polymeric structure with octahedral Fe(II) centers interconnected by acetate ligands. It is a coordination polymer.
Uses
Ferrous acetate is used as a mordant by the dye industry. Ebonizing wood is one such process.
References
- ^ Weber, Birgit; Betz, Richard; Bauer, Wolfgang; Schlamp, Stephan (2011). "Crystal Structure of Iron(II) Acetate". Zeitschrift für anorganische und allgemeine Chemie. 637: 102–107. doi:10.1002/zaac.201000274.
- ^ Lide, David R., ed. (2009). CRC Handbook of Chemistry and Physics (90th ed.). Boca Raton, Florida: CRC Press. ISBN 978-1-4200-9084-0.
- ^ Sigma-Aldrich Co., Iron(II) acetate. Retrieved on 2014-05-03.
- "MSDS of Ferrous acetate". fishersci.ca. Fair Lawn: Fisher Scientific. Retrieved 2014-08-02.
- "Synthesis of Iron(II) acetate hydrate (ferrous acetate)". Archived from the original on 2013-08-25. Retrieved 2009-01-07.
- Wildermuth, Egon; Stark, Hans; Friedrich, Gabriele; Ebenhöch, Franz Ludwig; Kühborth, Brigitte; Silver, Jack; Rituper, Rafael (2000). "Iron Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a14_591. ISBN 978-3527306732.
- Ebonizing Wood with Ferric Acetate
| Iron compounds | |||
|---|---|---|---|
| Fe(−II) | |||
| Fe(0) | |||
| Fe(I) |
| ||
| Fe(0,II) | |||
| Fe(II) |
| ||
| Fe(0,III) | |||
| Fe(II,III) | |||
| Fe(III) |
| ||
| Fe(IV) | |||
| Fe(VI) | |||
| Purported | |||
| sort | |||
| Acetyl halides and salts of the acetate ion | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||