 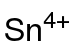
| |
| Names | |
|---|---|
| Other names
Tin(IV) acetate Tin tetraacetate | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.157.007 |
| EC Number |
|
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | Sn(CH3COO)4 |
| Molar mass | 353.89 |
| Appearance | white needles |
| Melting point | 242 °C (468 °F; 515 K) |
| Hazards | |
| GHS labelling: | |
| Pictograms | 
|
| Signal word | Warning |
| Hazard statements | H302, H312, H332 |
| Precautionary statements | P261, P264, P270, P271, P280, P301+P317, P302+P352, P304+P340, P317, P321, P330, P362+P364, P501 |
| Related compounds | |
| Other anions | Tin(IV) fluoroacetate |
| Other cations | Lead(IV) acetate |
| Related compounds | Tin(II) acetate |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Tin(IV) acetate is the acetate salt of tin(IV), with the chemical formula of Sn(CH3COO)4.
Preparation
Tin(IV) acetate can be refluxed by thallium acetate and tin(IV) iodide in acetic anhydride. After the reaction is completed, the solution is concentrated and cooled to precipitate crystals, which are washed with anhydrous ether and dried in vacuum:
- 4 CH3COOTl + SnI4 → Sn(CH3COO)4 + 4 TlI↓
Tetraphenyltin is refluxed at 120 °C in acetic acid-acetic anhydride mixture, and tin(IV) acetate can be quantitatively generated:
- 4 CH3COOH + (C6H5)4Sn → Sn(CH3COO)4 + 4C6H6
The reaction of tin(IV) nitrate with acetic acid and acetic anhydride can also produce tin(IV) acetate, but the reaction with trifluoroacetic anhydride can not get its analogue, but (NO2)2.
- 4 CH3COOH + Sn(NO3)4 → Sn(CH3COO)4 + 4 HNO3
Properties
Tin(IV) acetate decomposes in water to form tin hydroxide and acetic acid:
- Sn(CH3COO)4 + 4 H2O → Sn(OH)4 + 4 CH3COOH
It reacts with sulfur-containing species such as thiols to generate corresponding sulfur-containing tin compounds.
See also
References
- ^ Tin(IV) acetate
- "Tin(IV) acetate". pubchem.ncbi.nlm.nih.gov.
- Sawyer, Albert K.; Frey, Craig (January 1983). "A Simple Synthesis of Tin(IV) Acetate from Tetraphenyltin". Synthesis and Reactivity in Inorganic and Metal-Organic Chemistry. 13 (2): 259–262. doi:10.1080/00945718308059330. ISSN 0094-5714.
- Harrison, Philip G.; Khalil, Mutassim I.; Logan, Norman (January 1978). "A contribution to the chemistry of tin(IV) nitrate". Inorganica Chimica Acta. 30: 165–170. doi:10.1016/S0020-1693(00)89031-3.
- Mehrotra, R.C.; Srivastava, G.; Vasanta, E.N. (January 1981). "Reactions of tin tetraacetate with sulphur ligands". Inorganica Chimica Acta. 47: 125–130. doi:10.1016/S0020-1693(00)89317-2.
Further reading
- Sakuntala, E. N.; Shanker, Rama (May 1986). "Reactions of tin tetraacetate with benzothiazolines". Monatshefte für Chemie Chemical Monthly. 117 (5): 607–612. doi:10.1007/BF00817897. ISSN 0026-9247. S2CID 92773170.
| Tin compounds | |
|---|---|
| Sn(II) | |
| Sn(IV) | |
| Acetyl halides and salts of the acetate ion | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||