Iron(II) phosphate
Names
IUPAC name
Iron(II) phosphate
Other names
Ferrous phosphate
Identifiers
CAS Number
3D model (JSmol )
ChemSpider
ECHA InfoCard
100.035.456
EC Number
PubChem CID
UNII
CompTox Dashboard (EPA )
InChI
InChI=1S/3Fe.2H3O4P/c;;;2*1-5(2,3)4/h;;;2*(H3,1,2,3,4)/q3*+2;;/p-6Key: SDEKDNPYZOERBP-UHFFFAOYSA-H
SMILES
Properties
Chemical formula
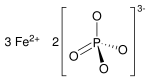
Fe3 (PO4 )2
Appearance
brown powder
Density
2.61 g/cm (octahydrate)
Melting point
180 °C (356 °F; 453 K) (octahydrate) decomposes
Solubility in water
insoluble
Structure
Crystal structure
monoclinic (octahydrate)
Space group
C 2/m
Lattice constant
a = 10.086 (octahydrate), b = 13.441 (octahydrate), c = 4.703 (octahydrate)α = 90°, β = 104.27°, γ = 90°
Hazards
GHS labelling
Pictograms
Signal word
Warning
Hazard statements
H315 , H319 , H335
Precautionary statements
P261 , P280 , P304+P340 , P305+P351+P338 , P405 , P501
NFPA 704
2
1
0
Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
Infobox references
Chemical compound
Iron(II) phosphate , also ferrous phosphate , Fe3 (PO4 )2 , is an iron salt of phosphoric acid .
Natural occurrences
The mineral vivianite is a naturally occurring form of hydrated iron(II) phosphate.
Production
It can be formed by the reaction of ferrous hydroxide with phosphoric acid to produce hydrated iron(II) phosphate.
See also
References
"iron(II) phosphate octahydrate" . chemister.ru . Retrieved 2 July 2014."Safety Data Sheet" . fishersci.com . Retrieved 12 August 2023."Iron(II) Phosphate" . EndMemo.com . Retrieved 22 January 2016.
External links
Iron(II) phosphate at Wikimedia Commons
Iron compounds Fe(−II)
Fe(0)
Fe(I)
Fe(0,II)
Fe(II)
Fe(0,III)
Fe(II,III)
Fe(III)
Organoiron(III) compounds
Fe(IV)
Fe(VI)
Purported
sort
Categories :
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.
**DISCLAIMER** We are not affiliated with Wikipedia, and Cloudflare.
The information presented on this site is for general informational purposes only and does not constitute medical advice.
You should always have a personal consultation with a healthcare professional before making changes to your diet, medication, or exercise routine.
AI helps with the correspondence in our chat.
We participate in an affiliate program. If you buy something through a link, we may earn a commission 💕
↑



![]() Media related to Iron(II) phosphate at Wikimedia Commons
Media related to Iron(II) phosphate at Wikimedia Commons