| |

| |
| Names | |
|---|---|
| Other names cobalt violet, cobalt(II) phosphate, cobalt orthophosphate, Pigment Violet 14 | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.033.309 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | Co3(PO4)2 |
| Molar mass | 366.74231 g/mol |
| Appearance | violet solid |
| Density | 3.81 g/cm |
| Melting point | 1,160 °C (2,120 °F; 1,430 K) |
| Solubility in water | insoluble |
| Solubility product (Ksp) | 2.05×10 |
| Magnetic susceptibility (χ) | 28,110.0·10 cm/mol |
| Refractive index (nD) | 1.7 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Cobalt phosphate is the inorganic compound with the formula Co3(PO4)2. It is a commercial inorganic pigment known as cobalt violet. Thin films of this material are water oxidation catalysts.

A swatch of cobalt violet, popular among the French impressionists.
Preparation and structure
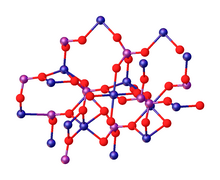
The tetrahydrate Co3(PO4)2•4H2O precipitates as a solid upon mixing aqueous solutions of cobalt(II) and phosphate salts. Upon heating, the tetrahydrate converts to the anhydrous material. According to X-ray crystallography, the anhydrous Co3(PO4)2 consists of discrete phosphate (PO
4) anions that link Co
centres. The cobalt ions occupy both octahedral (six-coordinate) and pentacoordinate sites in a 1:2 ratio.
See also
References
- John Rumble (June 18, 2018). CRC Handbook of Chemistry and Physics (99th ed.). CRC Press. pp. 5–188. ISBN 978-1138561632.
- Hugo Müller, Wolfgang Müller, Manfred Wehner, Heike Liewald "Artists' Colors" in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a03_143.pub2
- Matthew W. Kanan; Yogesh Surendranatha; Daniel G. Nocera (2009). "Cobalt–phosphate oxygen-evolving Compound". Chem. Soc. Rev. 38 (1): 109–114. doi:10.1039/B802885K. PMID 19088970.
- Donaldson, John Dallas; Beyersmann, Detmar (2005). "Cobalt and Cobalt Compounds". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a07_281.pub2. ISBN 9783527303854.
- Sankar, Selvasundarasekar Sam; Rathishkumar, Arumugam; Geetha, Kathiresan; Kundu, Subrata (2020-10-15). "A Simple Route for the Synthesis of Cobalt Phosphate Nanoparticles for Electrocatalytic Water Oxidation in Alkaline Medium". Energy & Fuels. 34 (10): 12891–12899. doi:10.1021/acs.energyfuels.0c02809. ISSN 0887-0624. S2CID 224960926.
- Anderson, J. B.; Kostiner, E.; Miller, M. C.; Rea, J. R. (1975). "Crystal structure of cobalt orthophosphate Co3(PO4)2". Journal of Solid State Chemistry. 14 (4): 372–377. Bibcode:1975JSSCh..14..372A. doi:10.1016/0022-4596(75)90058-4.
- Nord, A. G.; Stefanidis, T. (1983). "Structure refinements of Co3(PO4)2. A Note on the Reliability of Powder Diffraction Studies". Acta Chemica Scandinavica A. 37: 715–721. doi:10.3891/acta.chem.scand.37a-0715.
| Cobalt compounds | |
|---|---|
| Cobalt(I) | |
| Cobalt(II) | |
| Cobalt(0,III) | |
| Cobalt(II,III) | |
| Cobalt(III) | |
| Cobalt(III,IV) | |
| Cobalt(IV) | |
| Cobalt(V) | |
| Phosphates | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||