
| |
| |
| Names | |
|---|---|
| IUPAC name Nickel(II) carbonate | |
| Other names Nickelous carbonate | |
| Identifiers | |
| CAS Number |
|
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.020.063 |
| EC Number |
|
| PubChem CID | |
| RTECS number |
|
| UN number | 3288 |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | NiCO3 |
| Molar mass | 118.7 |
| Appearance | light green powder |
| Density | 4.39 g/cm |
| Melting point | 205 °C (401 °F; 478 K) decomposes |
| Solubility in water | 0.0093 g/100ml |
| Solubility product (Ksp) | 6.6·10 |
| Structure | |
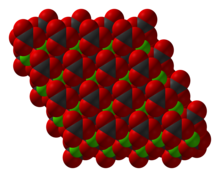
| Crystal structure | rhombohedral |
| Hazards | |
| GHS labelling: | |
| Pictograms |  
|
| Signal word | Danger |
| Hazard statements | H302, H312, H315, H317, H319, H332, H334, H335, H350 |
| Precautionary statements | P201, P261, P280, P305+P351+P338, P308+P313 |
| NFPA 704 (fire diamond) |
 |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) | 840 mg/kg |
| Safety data sheet (SDS) | ICSC 0927 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Nickel(II) carbonate describes one or a mixture of inorganic compounds containing nickel and carbonate. From the industrial perspective, an important nickel carbonate is basic nickel carbonate with the formula Ni4CO3(OH)6(H2O)4. Simpler carbonates, ones more likely encountered in the laboratory, are NiCO3 and its hexahydrate. All are paramagnetic green solids containing Ni cations. The basic carbonate is an intermediate in the hydrometallurgical purification of nickel from its ores and is used in electroplating of nickel.
Preparation
The hexahydrate NiCO3•6H2O is claimed to form upon electrolysis of nickel metal under an atmosphere of carbon dioxide. Green and yellow forms of anhydrous NiCO3 form when aqueous nickel chloride solutions are heated under high pressures of carbon dioxide.
Structure and reactions
NiCO3 adopts a structure like calcite, consisting of nickel in an octahedral coordination geometry. A pentahydrate has also been characterized by X-ray crystallography. Also known as the mineral hellyerite, the solid consists of subunits with an extra water of hydration.
Nickel carbonates are hydrolyzed upon contact with aqueous acids to give solutions containing the ion , liberating water and carbon dioxide in the process. Calcining (heating to drive off CO2 and water) of these carbonates gives nickel(II) oxide:
The nature of the resulting oxide depends on the nature of the precursor. The oxide obtained from the basic carbonate is often most useful for catalysis.
Basic nickel carbonate can be made by treating solutions of nickel sulfate with sodium carbonate:
The hydrated carbonate has been prepared by electrolytic oxidation of nickel in the presence of carbon dioxide:
Uses
Nickel carbonates are used in some ceramic applications and as precursors to catalysts.
Natural occurrence
The natural nickel carbonate is hellyerite, mentioned above. Basic Ni carbonates also have some natural representatives.
References
- https://www.conncoll.edu/media/website-media/offices/ehs/envhealthdocs/Nickel_Carbonate.pdf
- ^ Sigma-Aldrich Co., Nickel(II) carbonate hydroxide tetrahydrate. Retrieved on 2014-05-06.
- Keith Lascelles, Lindsay G. Morgan, David Nicholls, Detmar Beyersmann, "Nickel Compounds" in Ullmann's Encyclopedia of Industrial Chemistry Wiley-VCH, Weinheim, 2005. doi:10.1002/14356007.a17_235.pub2
- O. Glemser (1963). "Nickel(II) Carbonate". In G. Brauer (ed.). Handbook of Preparative Inorganic Chemistry, 2nd Ed. Vol. 2pages=1557-9. NY,NY: Academic Press.
- Pertlik, F. (1986). "Structures of hydrothermally synthesized cobalt(II) carbonate and nickel(II) carbonate". Acta Crystallographica Section C. 42 (1): 4–5. Bibcode:1986AcCrC..42....4P. doi:10.1107/S0108270186097524.
- Bette, Sebastian; Rincke, Christine; Dinnebier, Robert E.; Voigt, Wolfgang (2016). "Crystal Structure and Hydrate Water Content of Synthetic Hellyerite, NiCO3·5.5H2O". Zeitschrift für Anorganische und Allgemeine Chemie. 642 (9–10): 652–659. doi:10.1002/zaac.201600044.
- Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. p. 1557.
- "Gaspéite".
| Compounds containing the carbonate group | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Nickel compounds | |
|---|---|
| Nickel(0) | |
| Nickel(II) | |
| Nickel(III) | |
| Nickel(IV) | |


