| |
| Names | |
|---|---|
| IUPAC name Praseodymium(III) carbonate | |
| Identifiers | |
| CAS Number |
|
| 3D model (JSmol) |
|
| EC Number |
|
| PubChem CID |
|
| CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
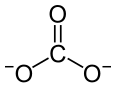
| Chemical formula | Pr2(CO3)3 |
| Molar mass | 461.849 (anhydrous) 605.977 (octahydrate) |
| Appearance | green crystals (octahydrate) |
| Solubility in water | insoluble (1.99×10mol/L) |
| Related compounds | |
| Other anions | Praseodymium(III) chloroacetate Praseodymium(III) sulfate Praseodymium(III) nitrate |
| Other cations | cerium carbonate neodymium carbonate |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Praseodymium(III) carbonate is an inorganic compound, with a chemical formula of Pr2(CO3)3. The anhydrous form is olive green, and many of its hydrates such as heptahydrate and octahydrate are known. They are all insoluble in water.
Preparation
Praseodymium(III) carbonate can be obtained by the hydrolysis of praseodymium(III) chloroacetate:
- 2 Pr(C2Cl3O2)3 + 3 H2O → Pr2(CO3)3 + 6 CHCl3 + 3 CO2
It can also be obtained by reacting sodium bicarbonate saturated with carbon dioxide with a praseodymium chloride solution.
Chemical properties
Praseodymium(III) carbonate is soluble in acids, and emits carbon dioxide:
- Pr2(CO3)3 + 6 H → 2 Pr + 3 H2O + 3 CO2↑
However, it is insoluble in water.
Other compounds
Praseodymium(III) carbonate forms compounds with N2H4, such as Pr2(CO3)3•12N2H4•5H2O which is a pale green crystal that is slightly soluble in water but insoluble in benzene, with d20°C = 1.873 g/cm.
References
- 《化学化工物性数据手册》 (无机卷) . 化学工业出版社. P320. 7.2 碳酸盐. ISBN 7-5025-3591-8
- ^ 《无机化学丛书》. 第七卷 钪 稀土元素. 易宪武 黄春晖 等编.科学出版社. P174. 碳酸盐. ISBN 978-7-03-030574-9
- Rare earth elements: Main volume, Phần 3 (Leopold Gmelin; Verlag Chemie, 1994), page 22; 68. Retrieved 4 Feb 2021.
- ^ 冯天泽. 稀土碳酸盐的制法、性质和组成. 《稀土》. 1989年第3期. pp.45~49
- PubChem. "Praseodymium Carbonate Octahydrate". pubchem.ncbi.nlm.nih.gov. Retrieved 2022-07-10.
- Uchenye zapiski: Serii︠a︡ khimicheskikh nauk (S.M. Kirov adyna Azărbai̐jan Dȯvlăt Universiteti; 1977), page 37. Retrieved 7 Feb 2021.
| Praseodymium compounds | |||
|---|---|---|---|
| Pr(II) | |||
| Pr(III) |
| ||
| Pr(III,IV) | |||
| Pr(IV) | |||
| Pr(V) | |||
| Compounds containing the carbonate group | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||