| |
| Names | |
|---|---|
| IUPAC name Praseodymium(IV) oxide | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.031.658 |
| EC Number |
|
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | PrO2 |
| Molar mass | 172.91 |
| Appearance | Dark brownish crystal |
| Structure | |
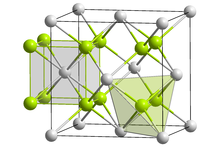
| Crystal structure | Fluorite structure |
| Space group | Fm3m (No. 225) |
| Lattice constant | a = 539.3 pm |
| Formula units (Z) | 4 |
| Related compounds | |
| Related compounds | Praseodymium(III) oxide Praseodymium(III,IV) oxide |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Praseodymium(IV) oxide is an inorganic compound with chemical formula PrO2.
Production
Praseodymium(IV) oxide can be produced by boiling Pr6O11 in water or acetic acid:
- Pr6O11 + 3 H2O → 4 PrO2 + 2 Pr(OH)3
Chemical reactions
Praseodymium(IV) oxide starts to decompose at 320~360 °C, liberating oxygen.
References
- ^ Chinese: 《无机化学丛书》.第七卷 钪 稀土元素. 科学出版社. 1.3.4 氧化态+4的化合物. P193~195
- Kern, Sanford (1964). "Magnetic Susceptibility of Praseodymium Oxides". The Journal of Chemical Physics. 40 (1). AIP Publishing: 208–212. Bibcode:1964JChPh..40..208K. doi:10.1063/1.1724864. ISSN 0021-9606.
| Praseodymium compounds | |||
|---|---|---|---|
| Pr(II) | |||
| Pr(III) |
| ||
| Pr(III,IV) | |||
| Pr(IV) | |||
| Pr(V) | |||