| |
| Identifiers | |
|---|---|
| CAS Number | |
| 3D model (JSmol) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | SSc |
| Molar mass | 77.02 g·mol |
| Appearance | gold-coloured solid |
| Structure | |
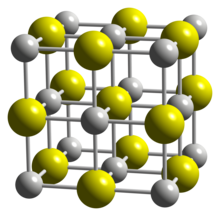
| Crystal structure | cubic |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Scandium monosulfide is a chemical compound of scandium and sulfur with the chemical formula ScS. Although its formula might suggest that it is a compound of scandium(II), i.e. , ScS is probably more realistically described as a pseudo-ionic compound, containing , with the remaining electron occupying the conduction band of the solid.
Structure
Scandium monosulfide adopts the sodium chloride crystal structure type.
Synthesis
Scandium monosulfide can be prepared by heating a mixture of scandium metal and powdered sulfur in the absence of air to 1150 °C for 70 hours.
- Sc + S → ScS
References
- ^ Dismukes, J. P.; White, J. G. (1964). "The Preparation, Properties, and Crystal Structures of Some Scandium Sulfides in the Range Sc2S3-ScS". Inorg. Chem. 3 (9): 1220–1228. doi:10.1021/ic50019a004.
| Scandium compounds | |
|---|---|
This inorganic compound–related article is a stub. You can help Misplaced Pages by expanding it. |