| |
| Names | |
|---|---|
| Other names Scandium monophosphide, phosphanylidynescandium | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.032.153 |
| EC Number |
|
| PubChem CID | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | ScP |
| Molar mass | 75.929670 g·mol |
| Structure | |
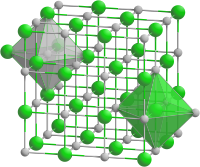
| Crystal structure | Rock salt structure |
| Space group | Fm3m |
| Lattice constant | a = 0.5312 nm |
| Formula units (Z) | 4 |
| Coordination geometry | Octahedral at Sc, Octahedral at P |
| Related compounds | |
| Other anions | |
| Other cations | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Scandium phosphide is an inorganic compound of scandium and phosphorus with the chemical formula ScP.
Synthesis
ScP can be obtained by the reaction of scandium and phosphorus at 1000 °C.
- 4 Sc + P4 → 4 ScP
Physical properties
This compound is calculated to be a semiconductor used in high power, high frequency applications and in laser diodes.
Chemical properties
ScP can be smelted with cobalt or nickel through electric arc to obtain ScCoP and ScNiP.
References
- Gschneidner (Jr.), Karl A.; Eyring, LeRoy (1978). Handbook on the Physics and Chemistry of Rare Earths: without special title. North-Holland Publishing Company. p. 287. ISBN 978-0-444-82507-0. Retrieved 10 December 2021.
- ^ Parthé, E. (10 January 1963). "Note on the structure of ScP and YP". Acta Crystallographica. 16 (1): 71. Bibcode:1963AcCry..16...71P. doi:10.1107/S0365110X63000141. Retrieved 12 December 2021.
- "Scandium Phosphide". American Elements. Retrieved 10 December 2021.
- "scandium phosphide". National Institute of Standards and Technology. Retrieved 10 December 2021.
- Toxic Substances Control Act (TSCA) Chemical Substance Inventory. U.S. Government Printing Office. 1979. p. 79. Retrieved 10 December 2021.
- Karil, Poornima; Karma, Nikita; Choudhary, K. K.; Kaurav, Netram (29 May 2020). "Effect of pressure on structural and elastic properties of Scandium phosphide". AIP Conference Proceedings. Emerging Interfaces of Physical Sciences and Technology 2019: Eipt2019. 2224 (1): 030001. Bibcode:2020AIPC.2224c0001K. doi:10.1063/5.0000475. S2CID 219883570. Retrieved 10 December 2021.
- Perkins, Peter G.; Marwaha, Ashok K.; Stewart, James J. P. (1 November 1981). "The band structures and magnetic properties of some transition-metal monophosphides I. Scandium phosphide". Theoretica Chimica Acta. 59 (6): 555–568. doi:10.1007/BF00552849. S2CID 94901262. Retrieved 10 December 2021.
- Kleinke, Holger; Franzen, Hugo F. (1 May 1998). "Sc–Sc Bonding in the New Ternary Phosphide ScNiP". Journal of Solid State Chemistry. 137 (2): 218–222. Bibcode:1998JSSCh.137..218K. doi:10.1006/jssc.1997.7704. Retrieved 12 December 2021.
| Scandium compounds | |
|---|---|
| Phosphorus compounds | |
|---|---|
| Phosphides | |
| Other compounds | |
| Phosphides | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Binary phosphides |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ternary phosphides | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Quaternary phosphides | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Quinary phosphides | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| See also | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||