| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name Selenium oxychloride | |||
| Other names Seleninyl chloride | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChemSpider | |||
| ECHA InfoCard | 100.029.313 | ||
| EC Number |
| ||
| PubChem CID | |||
| RTECS number |
| ||
| UNII | |||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
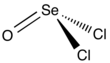
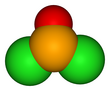
| Chemical formula | SeOCl2 | ||
| Molar mass | 165.87 g/mol | ||
| Appearance | colorless liquid | ||
| Density | 2.43 g/cm, liquid | ||
| Melting point | 10.9 °C (51.6 °F; 284.0 K) | ||
| Boiling point | 177.2 °C (351.0 °F; 450.3 K) | ||
| Refractive index (nD) | 1.651 (20 °C) | ||
| Structure | |||
| Molecular shape | trigonal pyramidal | ||
| Hazards | |||
| GHS labelling: | |||
| Pictograms |    
| ||
| Signal word | Warning | ||
| Hazard statements | H301, H314, H331, H373, H410 | ||
| Precautionary statements | P260, P261, P264, P270, P271, P273, P280, P301+P310, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P311, P314, P321, P330, P363, P391, P403+P233, P405, P501 | ||
| NFPA 704 (fire diamond) |
 | ||
| Lethal dose or concentration (LD, LC): | |||
| LDLo (lowest published) | 2 mg/kg (rabbit, dermal) | ||
| Related compounds | |||
| Related compounds | SOCl2, POCl3 | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Selenium oxydichloride is the inorganic compound with the formula SeOCl2. It is a colorless liquid. With a high dielectric constant (55) and high specific conductance, it is an attractive solvent. Structurally, it is a close chemical relative of thionyl chloride SOCl2, being a pyramidal molecule.
Preparation and reactions
Selenium oxydichloride can be prepared by several methods, and a common one involves the conversion of selenium dioxide to dichloroselenious acid followed by dehydration:
- SeO2 + 2 HCl → Se(OH)2Cl2
- Se(OH)2Cl2 → SeOCl2 + H2O
The original synthesis involved the redistribution reaction of selenium dioxide and selenium tetrachloride.
Pure selenium oxydichloride autoionizes to a dimer:
- SeOCl2 ↔ (SeO)2Cl
3 + Cl
The SeOCl2 is generally a labile Lewis acid and solutions of sulfur trioxide in SeOCl2 likely form the same way.
The compound hydrolyzes readily to form hydrogen chloride and selenium dioxide, and very few organic compounds dissolve in it without reaction. At elevated temperatures, it is a strong oxidizer, yielding a chloride, selenium dioxide, and diselenium dichloride.
See also
- Selenium oxybromide SeOBr2
- Selenous acid H2SeO3
References
- "Selenium compounds (as Se)". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- "Selenium oxychloride". pubchem.ncbi.nlm.nih.gov. Retrieved 12 December 2021.
- Smith, G. B. L.; Jackson, Julius (1950). "Selenium(IV) Oxychloride". Inorganic Syntheses. Vol. 3. pp. 130–137. doi:10.1002/9780470132340.ch34. ISBN 9780470132340.
- Audrieth & Kleinberg 1953, p. 237.
- Audrieth & Kleinberg 1953, pp. 239–242.
- Audrieth, Ludwig F.; Kleinberg, Jacob (1953). Non-aqueous solvents. New York: John Wiley & Sons. pp. 235–6. LCCN 52-12057.
| Selenium compounds | |
|---|---|
| Se(−II) | |
| Se(0,I) | |
| Se(I) | |
| Se(II) | |
| Se(IV) | |
| Se(VI) | |
| Se(IV,VI) | |
This inorganic compound–related article is a stub. You can help Misplaced Pages by expanding it. |