 | |
| Clinical data | |
|---|---|
| Other names | 2--1,3-thiazole-4-carboxamide |
| ATC code | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
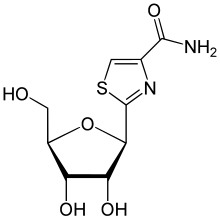
| Formula | C9H12N2O5S |
| Molar mass | 260.26 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (what is this?) (verify) | |
Tiazofurin is a drug which acts as an inhibitor of the enzyme IMP dehydrogenase. Tiazofurin and its analogues were under investigation for potential use in the treatment of cancer, though side effects such as pleuropericarditis and a flu-like syndrome precluded further development. They also show antiviral effects and may be reevaluated as potential options in the treatment of newly emerging viral diseases.
Synthesis

The treatment of 1-O-Acetyl-2,3,5-tri-O-benzoyl-beta-D-ribofuranose (1) with trimethylsilyl cyanide gives 2,3,5-Tri-O-benzoyl-beta-D-ribofuranosyl cyanide (2). Treatment with hydrogen sulfide led to (2R,3R,4R,5R)-2-((Benzoyloxy)methyl)-5-carbamothioyltetrahydrofuran-3,4-diyl dibenzoate, PC10907289 (3). Cyclization with Ethyl bromopyruvate (4) led to 2-(2,3,5-Tri-O-benzoyl-beta-D-ribofuranosyl)-4-thiazolecarboxylic Acid Ethyl Ester (5). Removal of the protecting groups with sodium methoxide afforded 2-beta-D-Ribofuranosyl-4-thiazolecarboxylic Acid Ethyl Ester (6). Amide-ester interchange by treatment with dry ammonia completed the synthesis of Tiazofurin (7).
References
- Popsavin M, Torović L, Svircev M, et al. (2006). "Synthesis and antiproliferative activity of two new tiazofurin analogues with 2'-amido functionalities". Bioorg. Med. Chem. Lett. 16 (10): 2773–6. doi:10.1016/j.bmcl.2006.02.001. PMID 16495053.
- De Clercq E (March 2016). "C-Nucleosides To Be Revisited". Journal of Medicinal Chemistry. 59 (6): 2301–11. doi:10.1021/acs.jmedchem.5b01157. PMID 26513594.
- Eastland, G.; Tiazofurine. Drugs Fut 1985, 10, 4, 304.
This antineoplastic or immunomodulatory drug article is a stub. You can help Misplaced Pages by expanding it. |