| Revision as of 03:07, 7 June 2009 edit206.74.169.116 (talk) →Models to explain homochiralityTag: references removed← Previous edit | Latest revision as of 00:15, 6 January 2025 edit undoZergTwo (talk | contribs)38 editsm Undid revision 1267610527 by 2607:FEA8:1B21:8D00:2987:F465:FA99:DD7C (talk) undoing edit per MOS:SECTIONHEADTag: Undo | ||
| Line 1: | Line 1: | ||
| {{Short description|Life arising from non-living matter}} | |||
| {{Redirect|Primordial soup|the board game|Primordial Soup (board game)}} | |||
| {{Redirect|Origin of life| |
{{Redirect|Origin of life |non-scientific<!--Please do not attempt to change this without obtaining wide consensus first, the wording has been carefully checked, thanks--> views on the origins of life|Creation myth}} | ||
| {{Good article}} | |||
| {{Use dmy dates |date=June 2020}} | |||
| {{Use American English |date=December 2019}} | |||
| ] and the abiotic synthesis of simple molecules, to the largely unknown, like the derivation of the ] (LUCA) with its complex molecular functionalities.<ref name="Walker Packard Cody 2017"/>]] | |||
| <!--Please do not change the lead paragraph without first discussing on the talk page.--> | |||
| {{pp-move-indef}} | |||
| '''Abiogenesis''' is the natural process by which ] arises from ], such as simple ]s. The prevailing scientific<!--Please do not insert "theory", "speculative", "doubtful", etc., you can be blocked for pushing your point of view.--> ] is that the transition from non-living to ] on Earth was not a single event, but a process of increasing complexity involving the formation of a ], the prebiotic synthesis of organic molecules, molecular ], ], ], and the emergence of ]s. The transition from non-life to life has never been observed experimentally, but many proposals have been made for different stages of the process.<!-- Please do not add refs here; this is a summary only. --> | |||
| The study of abiogenesis aims to determine how pre-life ]s gave rise to life under conditions strikingly different from those on Earth today. It primarily uses tools from ] and ], with more recent approaches attempting a synthesis of many sciences. Life functions through the specialized chemistry of ] and water, and builds largely upon four key families of chemicals: ]s for cell membranes, ]s such as sugars, ]s for protein metabolism, and ] ] and ] for the mechanisms of heredity. Any successful theory of abiogenesis must explain the origins and interactions of these classes of molecules. | |||
| ] ] in the Siyeh Formation, ]. In 2002, William Schopf of ] published a paper in the ] '']'' arguing that geological formations such as this possess 3.5 ] (billion years old) ] ] microbes. If true, they would be the earliest known ].]] | |||
| Many approaches to abiogenesis investigate how self-replicating molecules, or their components, came into existence. Researchers generally think that current life descends from an ], although other self-replicating and self-catalyzing molecules may have preceded RNA. <!-- Please do not add refs here, this is a summary only -->Other approaches (]) focus on understanding how ] in chemical systems on the early Earth might have provided the ] necessary for self-replication. The classic 1952 ] demonstrated that most amino acids, the chemical constituents of ]s, can be synthesized from ]s under conditions intended to replicate those of the ]. External sources of energy may have triggered these reactions, including ], ], atmospheric entries of micro-meteorites, and implosion of bubbles in sea and ocean waves. <!-- Please do not add refs here, this is a summary only --> | |||
| In the ], '''abiogenesis''', or '''origin of life''', is the study of how ]<!-- "Life on Earth" is synonym for ] --> could have arisen from inanimate matter. It should not be confused with ], which is the study of how groups of living things change over time. ]s, often called "the building blocks of life", can form via natural chemical reactions unrelated to life, as demonstrated in the ], which involved simulating the conditions of the early Earth. In all living things, these amino acids are organized into ]s, and the construction of these proteins is mediated by ]s. Thus the question of how life on Earth originated is a question of how the first nucleic acids arose. | |||
| While the ] of all modern organisms (LUCA) is thought to have been quite different from the origin of life, investigations into LUCA can guide research into early universal characteristics. A ] approach has sought to characterize LUCA by identifying the genes shared by ] and ], members of the two major branches of life (with ]s included in the archaean branch in the ]). It appears there are 60 proteins common to all life and ] that trace to LUCA; their functions imply that the LUCA was ] with the ], deriving energy by ], and maintaining its hereditary material with DNA, the ], and ]s. Although the LUCA lived over 4 billion years ago (4 ]), researchers believe it was far from the first form of life. Earlier cells might have had a leaky membrane and been powered by a naturally occurring ] near a deep-sea white smoker ].<!-- Please do not add refs here, this is a summary only --> | |||
| The first living things on Earth are thought to be ] ]s. The oldest ancient fossil microbe-like objects are dated to be 3.5 ] (billion years old), just a few hundred million years younger than Earth itself.<ref name="Wilde2001">{{cite journal |author=Wilde SA, Valley JW, Peck WH, Graham CM |title=Evidence from detrital zircons for the existence of continental crust and oceans on the Earth 4.4 Gyr ago |journal=Nature |volume=409 |issue=6817 |pages=175–8 |year=2001 |month=January |pmid=11196637 |doi=10.1038/35051550 |url=}}</ref><ref name="Schopf2002">{{cite journal |author=Schopf JW, Kudryavtsev AB, Agresti DG, Wdowiak TJ, Czaja AD |title=Laser--Raman imagery of Earth's earliest fossils |journal=Nature |volume=416 |issue=6876 |pages=73–6 |year=2002 |month=March |pmid=11882894 |doi=10.1038/416073a |url=}}</ref> By 2.4 Ga, the ratio of stable ] of ], ] and ] shows the action of living things on inorganic minerals and sediments<ref name="Hayes2006">{{cite journal |last=Hayes |first=John M. |authorlink= |coauthors=Waldbauer, Jacob R. |year=2006 |month= |title= The carbon cycle and associated redox processes through time |journal=] |volume=361 |issue=1470 |pages=931–950 |doi=10.1098/rstb.2006.1840 }}</ref><ref name="Archer2006">{{cite journal |last=Archer |first=Corey |authorlink= |coauthors=Vance, Derek |year=2006 |month= |title=Coupled Fe and S isotope evidence for Archean microbial Fe(III) and sulfate reduction |journal=Geology |volume=34 |issue=3 |pages=153–156 |doi=10.1130/G22067.1 }}</ref> and molecular biomarkers indicate ], demonstrating that life on Earth was widespread by this time.<ref>{{cite journal |last=Cavalier-Smith |first=Thomas |authorlink= |coauthors=Brasier, Martin; Embley, T. Martin |year=2006 |month= |title=Introduction: how and when did microbes change the world? |journal=Phil. Trans. R. Soc. B |volume=361 |issue=1470 |pages=845–50 |doi=10.1098/rstb.2006.1847 }}</ref><ref>{{cite journal |last=Summons |first=Roger E. |authorlink= |coauthors=''et al.'' |year=2006 |month= |title=Steroids, triterpenoids and molecular oxygen |journal=Phil. Trans. R. Soc. B |volume=361 |issue=1470 |pages=951–68 |doi=10.1098/rstb.2006.1837 }}</ref> | |||
| Earth remains the only place in the ] known to harbor life. ] and ] from the Earth informs most studies of abiogenesis. The ] was formed at 4.54 Gya, and the earliest evidence of life on Earth dates from at least 3.8 Gya from ]. Some studies have suggested that ] may have lived within hydrothermal vent precipitates dated 3.77 to 4.28 Gya ], soon after ] 4.4 Gya during the ].<!-- Please do not add refs here, this is a summary only --> | |||
| On the other hand, the exact sequence of chemical events that led to the first nucleic acids is not known. Several hypotheses about ] have been proposed, most notably the ] (] without ]) and the ] (RNA life-forms). | |||
| == Overview == | |||
| ==History of the concept in science== | |||
| ===Spontaneous generation=== | |||
| {{main|Spontaneous generation}} | |||
| Until the early 19th century, people generally believed in the ongoing ] of certain forms of ] from non-living matter. This was paired with heterogenesis, beliefs where one form of life derives from a different form (''e.g.'' bees from flowers).<ref>{{cite book | editor = Philip P. Wiener | title = Dictionary of the History of Ideas | url = http://etext.virginia.edu/DicHist/dict.html | accessdate = 2009-01-24 | year = 1973 | publisher = Charles Scribner's Sons | location = New York | chapter = Spontaneous Generation | chapterurl = http://etext.virginia.edu/cgi-local/DHI/dhi.cgi?id=dv4-39}}</ref> Classical notions of abiogenesis, now more precisely known as ''spontaneous generation'', held that certain complex, living ]s are generated by decaying organic substances. According to ] it was a readily observable truth that ]s arise from the dew which falls on plants, ]s from putrid matter, mice from dirty hay, crocodiles from rotting logs at the bottom of bodies of water, and so on.<ref>{{cite book | |||
| | last = Lennox | |||
| | first = James | |||
| | title = Aristotle's Philosophy of Biology: Studies in the Origins of Life Science | |||
| | publisher = Cambridge Press | |||
| | date = 2001 | |||
| | location = New York, NY | |||
| | pages = 229–258 | |||
| | isbn = 978-0521659765}}</ref> | |||
| {{Further|Astrobiology}} | |||
| In the 17th century, such assumptions started to be questioned; for example, in 1646, ] published his '']'' (subtitled ''Enquiries into Very many Received Tenets, and Commonly Presumed Truths''), which was an attack on false beliefs and "vulgar errors." His conclusions were not widely accepted. For example, his contemporary, ] wrote: "To question this (i.e., spontaneous generation) is to question reason, sense and experience. If he doubts of this let him go to ], and there he will find the fields swarming with mice, begot of the mud of ], to the great calamity of the inhabitants."<ref>{{cite journal |last=Balme |first=D. M. |authorlink= |coauthors= |year=1962 |month= |title=Development of Biology in Aristotle and Theophrastus: Theory of Spontaneous Generation |journal=Phronesis: a journal for Ancient Philosophy |volume=7 |issue=1–2 |pages=91–104 |doi=10.1163/156852862X00052 }}</ref> | |||
| ] aimed to solve the puzzle of the origin of life – how a fully functioning living system could emerge from non-living components – through research on the prebiotic origin of ], both in ] and on ]s, as well as the functioning of early biomolecules to ] reactions and support ].<ref name="NASA strategy 2015"/>]] | |||
| In 1665, ] published the first drawings of a microorganism. Hooke was followed in 1676 by ], who drew and described microorganisms that are now thought to have been ] and ].<ref>{{cite book |title=Antony Van Leeuwenhoek and his little animals |author=Dobell, C. |year=1960 |publisher=Dover Publications |location=New York }}</ref> Many felt the existence of microorganisms was evidence in support of spontaneous generation, since microorganisms seemed too simplistic for ], and ] through ] had not yet been observed. | |||
| ] consists of reproduction with (heritable) variations.<ref name="JBSD-20110317">{{cite journal |last=Trifonov |first=Edward N. |author-link=Edward Trifonov |title=Vocabulary of Definitions of Life Suggests a Definition |date=17 March 2011 |journal=Journal of Biomolecular Structure and Dynamics |volume=29 |issue=2 |pages=259–266 |doi=10.1080/073911011010524992 |pmid=21875147 |s2cid=38476092 |doi-access=free |issn=0739-1102 }}</ref> ] defines life as "a self-sustaining chemical system capable of evolution]]."<ref name="NASA-20210306">{{cite web |last=Voytek |first=Mary A. |author-link=Mary Voytek |title=About Life Detection |url=https://astrobiology.nasa.gov/research/life-detection/about/ |date=6 March 2021 |publisher=] |access-date=8 March 2021 |archive-date=16 August 2021 |archive-url=https://web.archive.org/web/20210816150806/https://astrobiology.nasa.gov/research/life-detection/about/ |url-status=live }}</ref> Such a system is complex; the ] (LUCA), presumably a single-celled organism which lived some 4 billion years ago, already had hundreds of ]s encoded in the ] ] that is universal today. That in turn implies a suite of cellular machinery including ], ], and ]s to translate the code into ]s. Those proteins included ]s to operate its ] via the ], and a ] to replicate its genetic material.<ref name="Witzany 2016"/><ref name="AB-20141208"/> | |||
| The first solid evidence against spontaneous generation came in 1668 from ], who proved that no ]s appeared in meat when flies were prevented from laying eggs. It was gradually shown that, at least in the case of all the higher and readily visible organisms, the previous sentiment regarding spontaneous generation was false. The alternative seemed to be ]: that every living thing came from a pre-existing living thing (''omne vivum ex ovo'', Latin for "every living thing from an egg"). | |||
| The challenge for abiogenesis (origin of life)<ref>{{cite book |last=Oparin |first=Aleksandr Ivanovich |author-link=Alexander Oparin |translator-last=Morgulis |translator-first=Sergius |orig-year=1938 |title=The Origin of Life |url=https://books.google.com/books?id=Jv8psJCtI0gC |edition=2 |location=Mineola, New York |publisher=Courier |date=2003 |isbn=978-0-486-49522-4 |access-date=16 June 2018 |archive-date=2 April 2023 |archive-url=https://web.archive.org/web/20230402201809/https://books.google.com/books?id=Jv8psJCtI0gC |url-status=live }}</ref><ref name=Pereto /><ref name="AST-20151218">Compare: {{cite journal |last=Scharf |first=Caleb |title=A Strategy for Origins of Life Research |date=18 December 2015 |journal=] |volume=15 |issue=12 |pages=1031–1042 |doi=10.1089/ast.2015.1113 |display-authors=etal |pmid=26684503 |pmc=4683543 |bibcode=2015AsBio..15.1031S |quote=What do we mean by the origins of life (OoL)? ... Since the early 20th century the phrase OoL has been used to refer to the events that occurred during the transition from non-living to living systems on Earth, i.e., the origin of terrestrial biology (Oparin, 1924; Haldane, 1929). The term has largely replaced earlier concepts such as abiogenesis (Kamminga, 1980; Fry, 2000).}}</ref> researchers is to explain how such a complex and tightly interlinked system could develop by evolutionary steps, as at first sight ] to enable it to function. For example, a cell, whether the LUCA or in a modern organism, copies its DNA with the DNA polymerase enzyme, which is in turn produced by translating the DNA polymerase gene in the DNA. Neither the enzyme nor the DNA can be produced without the other.<ref name="Weiss Sousa Mrnjavac 2016"/> The evolutionary process could have involved molecular ], ] such as of ]s, and ] via RNA ]s.<ref name="Witzany 2016">{{cite journal |last=Witzany |first=Guenther |title=Crucial steps to life: From chemical reactions to code using agents |journal=] |year=2016 |volume=140 |pages=49–57 |url=http://www.biocommunication.at/pdf/publications/biosystems_2016.pdf |doi=10.1016/j.biosystems.2015.12.007 |pmid=26723230 |bibcode=2016BiSys.140...49W |s2cid=30962295 |access-date=30 October 2018 |archive-date=31 October 2018 |archive-url=https://web.archive.org/web/20181031052532/http://www.biocommunication.at/pdf/publications/biosystems_2016.pdf |url-status=live }}</ref><ref name="AB-20141208">{{cite web |last=Howell |first=Elizabeth |title=How Did Life Become Complex, And Could It Happen Beyond Earth? |url=https://www.astrobio.net/origin-and-evolution-of-life/life-become-complex-happen-beyond-earth/ |date=8 December 2014 |work=] |access-date=14 April 2022 |url-status=usurped |archive-url=https://web.archive.org/web/20180215024231/https://www.astrobio.net/origin-and-evolution-of-life/life-become-complex-happen-beyond-earth/ |archive-date=15 February 2018}}</ref><ref name="EA-20150420">{{cite book |last=Tirard |first=Stephane |title=Encyclopedia of Astrobiology |chapter=Abiogenesis |date=20 April 2015 |doi=10.1007/978-3-642-27833-4_2-4 |isbn=978-3-642-27833-4 |page=1 |quote=Thomas Huxley (1825–1895) used the term abiogenesis in an important text published in 1870. He strictly made the difference between spontaneous generation, which he did not accept, and the possibility of the evolution of matter from inert to living, without any influence of life. ... Since the end of the nineteenth century, evolutive abiogenesis means increasing complexity and evolution of matter from inert to living state in the abiotic context of evolution of primitive Earth.}}</ref> Nonetheless, the transition of non-life to life has never been observed experimentally, nor has there been a satisfactory chemical explanation.<ref>{{cite book |last1=Luisi |first1=Pier Luigi |title=The Emergence of Life: From Chemical Origins to Synthetic Biology |date=2018 |publisher=] |isbn=9781108735506 |page=416 |quote=However, the turning point of non-life to life has never been put into one experimental set up. There are, of course, several hypotheses, and this plethora of ideas means already that we do not have a convincing one.}}</ref> | |||
| In 1768, ] demonstrated that ]s were present in the air, and could be killed by boiling. In 1861, ] performed a series of experiments which demonstrated that organisms such as bacteria and fungi do not spontaneously appear in sterile, nutrient-rich media. | |||
| The preconditions to the development of a living cell like the LUCA are clear enough, though disputed in their details: a habitable world is formed with a supply of minerals and liquid water. Prebiotic synthesis creates a range of simple organic compounds, which are assembled into polymers such as proteins and RNA. On the other side, the process after the LUCA is readily understood: biological evolution caused the development of a wide range of species with varied forms and biochemical capabilities. However, the derivation of living things such as LUCA from simple components is far from understood.<ref name="Walker Packard Cody 2017">{{cite journal |last1=Walker |first1=Sara I. |last2=Packard |first2=N. |last3=Cody |first3=G. D. |title=Re-conceptualizing the origins of life |journal=] |volume=375 |issue=2109 |date=13 November 2017 |doi=10.1098/rsta.2016.0337 |page=20160337 |pmid=29133439 |pmc=5686397 |bibcode=2017RSPTA.37560337W}}</ref> | |||
| ===Pasteur and Darwin=== | |||
| By the middle of the 19th century, the theory of ] had accumulated so much evidential support, due to the work of ] and others, that the alternative theory of spontaneous generation had been effectively disproven. Pasteur himself remarked, after a definitive finding in 1864, "Never will the doctrine of spontaneous generation recover from the mortal blow struck by this simple experiment."<ref> | |||
| {{cite book | |||
| |title=Origin of Life | |||
| |last=Oparin | |||
| |first=Aleksandr I. | |||
| |date=1953 | |||
| |pages=p.196 | |||
| |publisher=Dover Publications, New York | |||
| |isbn=0486602133 | |||
| }} | |||
| </ref> | |||
| The collapse of spontaneous generation, however, left a vacuum of scientific thought on the question of how life ''had'' first arisen. | |||
| Although Earth remains the only place where life is known,<ref name="NASA-1990">{{cite journal |url=https://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/19900013148.pdf |title=Extraterrestrial Life in the Universe |last=Graham |first=Robert W. |date=February 1990 |location=], Cleveland, Ohio |website=] |type=NASA Technical Memorandum 102363 |access-date=2015-06-02 |url-status=live |archive-url=https://web.archive.org/web/20140903100534/http://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/19900013148.pdf |archive-date=3 September 2014}}</ref><ref>{{harvnb|Altermann|2009|p=xvii}}</ref> the science of ] seeks evidence of life on other planets. The 2015 NASA strategy on the origin of life aimed to solve the puzzle by identifying interactions, intermediary structures and functions, energy sources, and environmental factors that contributed to the diversity, selection, and replication of evolvable macromolecular systems,<ref name="NASA strategy 2015">{{cite web |title=NASA Astrobiology Strategy |year=2015 |work=NASA |access-date=24 September 2017 |url=https://nai.nasa.gov/media/medialibrary/2015/10/NASA_Astrobiology_Strategy_2015_151008.pdf |archive-url=https://web.archive.org/web/20161222190306/https://nai.nasa.gov/media/medialibrary/2015/10/NASA_Astrobiology_Strategy_2015_151008.pdf |archive-date=22 December 2016}}</ref> and mapping the chemical landscape of potential primordial informational ]s. The advent of polymers that could replicate, store genetic information, and exhibit properties subject to selection was, it suggested, most likely a critical step in the ] of prebiotic chemical evolution.<ref name="NASA strategy 2015"/> Those polymers derived, in turn, from simple ]s such as ]s, ]s, and ]s that could have been formed by reactions in the environment.<ref name=Oparin>{{harvnb|Oparin|1953|p=vi}}</ref><ref name=Pereto>{{cite journal |last=Peretó |first=Juli |year=2005 |title=Controversies on the origin of life |url=http://www.im.microbios.org/0801/0801023.pdf |journal=] |volume=8 |issue=1 |pages=23–31 |pmid=15906258 |access-date=1 June 2015 |archive-url=https://web.archive.org/web/20150824074726/http://www.im.microbios.org/0801/0801023.pdf |archive-date=24 August 2015}}</ref><ref>{{cite journal |last1=Warmflash |first1=David |last2=Warmflash |first2=Benjamin |date=November 2005 |title=Did Life Come from Another World? |journal=] |volume=293 |issue=5 |pages=64–71 |doi=10.1038/scientificamerican1105-64 |pmid=16318028 |bibcode=2005SciAm.293e..64W}}</ref><ref>{{harvnb|Yarus|2010|p=47}}</ref> A successful theory of the origin of life must explain how all these chemicals came into being.<ref>{{cite book |last1=Ward |first1=Peter |last2=Kirschvink |first2=Joe |author2-link=Joseph Kirschvink |date=2015 |title=A New History of Life: the radical discoveries about the origins and evolution of life on earth |publisher=] |pages=39–40 |isbn=978-1-60819-910-5}}</ref> | |||
| In a letter to ] on February 1, 1871,<ref> windmillministries.org, Retrieved on ]</ref> ] addressed the question, suggesting that the original spark of life may have begun in a "warm little pond, with all sorts of ammonia and phosphoric salts, lights, heat, electricity, etc. present, so that a protein compound was chemically formed ready to undergo still more complex changes". He went on to explain that "at the present day such matter would be instantly devoured or absorbed, which would not have been the case before living creatures were formed."<ref>"It is often said that all the conditions for the first production of a living organism are now present, which could ever have been present. But if (and oh! what a big if!) we could conceive in some warm little pond, with all sorts of ammonia and phosphoric salts, light, heat, electricity, &c., present, that a proteine compound was chemically formed ready to undergo still more complex changes, at the present day such matter would be instantly devoured or absorbed, which would not have been the case before living creatures were formed." written in 1871, published in ], ed. 1887. ''The life and letters of Charles Darwin, including an autobiographical chapter.'' London: John Murray. Volume 3. p. </ref> In other words, the presence of life itself makes the search for the origin of life dependent on the sterile conditions of the laboratory. | |||
| == Pre-1960s conceptual history == | |||
| ===Haldane and Oparin: The Primordial Soup Theory=== | |||
| {{further|]}} | |||
| ] (right) at the laboratory]] | |||
| {{Main|History of research into the origin of life}} | |||
| No new notable research or theory on the subject appeared until 1924, when ] (Aleksandr I. Oparin) reasoned that atmospheric oxygen prevents the synthesis of certain organic compounds that are necessary building blocks for the evolution of life. In his ''The Origin of Life'',<ref>Oparin, A. I. (1924) Proiskhozhozhdenie zhizny, Moscow (Translated by Ann Synge in Bernal (1967), The Origin of Life, Weidenfeld and Nicolson, London, pages 199-234.</ref><ref>{{cite book |title=The Origin of Life |author=Oparin, A. I. |year=1952 |publisher=Dover |location=New York }}</ref> Oparin proposed that the "spontaneous generation of life" that had been attacked by ], did in fact occur once, but was now impossible because the conditions found in the early earth had changed, and the presence of living organisms would immediately consume any spontaneously generated organism. Oparin argued that a "primeval soup" of organic molecules could be created in an oxygen-less atmosphere through the action of sunlight. These would combine in ever-more complex fashions until they formed ] droplets. These droplets would "]" by fusion with other droplets, and "]" through fission into daughter droplets, and so have a primitive ] in which those factors which promote "cell integrity" survive, those that do not become extinct. Many modern theories of the origin of life still take Oparin's ideas as a starting point. | |||
| ] was a synthesis of small organic molecules in a mixture of simple gases in a thermal gradient created by heating (right) and cooling (left) the mixture at the same time, with electrical discharges.]] | |||
| Around the same time, ] suggested that the Earth's pre-biotic oceans–very different from their modern counterparts–would have formed a "hot dilute soup" in which organic compounds could have formed. This idea was called ''biopoiesis'' or ''biopoesis'', the process of living matter evolving from self-replicating but nonliving molecules.<ref>{{cite book |title=Origins of Life |author=Bernal, J.D. |year=1969 |publisher=Wiedenfeld and Nicholson |location=London }}</ref><ref>{{cite book |author=Bryson, Bill |title=A short history of nearly everything |publisher=Black Swan |location=London |year=2004 |pages=300–2 |isbn=0-552-99704-8 }}</ref> | |||
| === Spontaneous generation === | |||
| ==Early conditions== | |||
| Morse and MacKenzie<ref>{{cite journal |last=Morse |first=J. W. |authorlink= |coauthors=MacKenzie, F. T. |year=1998 |title=Hadean Ocean Carbonate chemistry |journal=Aquatic Geochemistry |volume=4 |pages=301–19 |doi=10.1023/A:1009632230875 }}</ref> have suggested that ] first in the ] era, as soon as 200 ] (million years) after the Earth was formed, in a hot {{convert|100|°C|°F}} ] environment, and that the ] of about 5.8 rose rapidly towards neutral. This has been supported by Wilde<ref name="Wilde2001" /> who has pushed the date of the ] crystals found in the metamorphosed ] of ] in Western Australia, previously thought to be 4.1–4.2 Ga, to 4.404 Ga. This means that oceans and ] existed within 150 Ma of Earth's formation. | |||
| {{Main|Spontaneous generation}} | |||
| Despite this, the ] environment was one highly hazardous to life. Frequent collisions with large objects, up to {{convert|500|km|mi}} in diameter, would have been sufficient to vaporise the ocean within a few months of impact, with hot steam mixed with rock vapour leading to high altitude clouds completely covering the planet. After a few months the height of these clouds would have begun to decrease but the cloud base would still have been elevated for about the next thousand years. After that, it would have begun to rain at low altitude. For another two thousand years rains would slowly have drawn down the height of the clouds, returning the oceans to their original depth only 3,000 years after the impact event.<ref>{{cite journal |last=Sleep |first=Norman H. |authorlink= |coauthors=''et al.'' |year=1989 |month= |title=Annihilation of ecosystems by large asteroid impacts on early Earth |journal=Nature |volume=342 |issue= |pages=139–142 |doi=10.1038/342139a0 }}</ref> | |||
| One ancient view of the origin of life, from ] until the 19th century, is of ].<ref>{{harvnb|Sheldon|2005}}</ref> This theory held that "lower" animals such as insects were generated by decaying organic substances, and that life arose by chance.<ref>{{harvnb|Lennox|2001|pp=229–258}}</ref><ref name="Bernal 1967">{{harvnb|Bernal|1967}}</ref> This was questioned from the 17th century, in works like ]'s '']''.<ref>{{cite journal |last=Balme |first=D. M. |author-link=David Mowbray Balme |year=1962 |title=Development of Biology in Aristotle and Theophrastus: Theory of Spontaneous Generation |journal=] |volume=7 |issue=1–2 |pages=91–104 |doi=10.1163/156852862X00052}}</ref><ref>{{harvnb|Ross|1652}}</ref> In 1665, ] published the first drawings of a ]. In 1676, ] drew and described microorganisms, probably ] and ].<ref>{{harvnb|Dobell|1960}}</ref> Van Leeuwenhoek disagreed with spontaneous generation, and by the 1680s convinced himself, using experiments ranging from sealed and open meat incubation and the close study of insect reproduction, that the theory was incorrect.<ref>{{harvnb|Bondeson|1999}}</ref> In 1668 ] showed that no ]s appeared in meat when flies were prevented from laying eggs.<ref name=lev>{{cite web |last1=Levine |first1=R. |last2=Evers |first2=C. |title=The Slow Death of Spontaneous Generation (1668-1859) |url=http://www.accessexcellence.org/RC/AB/BC/Spontaneous_Generation.php |access-date=18 April 2013 |archive-url=https://web.archive.org/web/20080426191204/http://www.accessexcellence.org/RC/AB/BC/Spontaneous_Generation.php |archive-date=26 April 2008}}</ref> By the middle of the 19th century, spontaneous generation was considered disproven.<ref>{{harvnb|Oparin|1953|p=196}}</ref><ref name="Tyndall Fragments2">{{harvnb|Tyndall|1905|loc=IV, XII (1876), XIII (1878)}}</ref> | |||
| Between 3.8 and 4.1 Ga, changes in the orbits of the ] planets may have caused a ] that pockmarked the moon and other inner planets (Mercury, Mars, and presumably Earth and Venus). This would likely have sterilized the planet had life appeared before that time. | |||
| === Panspermia === | |||
| By examining the time interval between such devastating environmental events, the time interval when life might first have come into existence can be found for different early environments. The study by Maher and Stephenson shows that if the deep marine hydrothermal setting provides a suitable site for the origin of life, abiogenesis could have happened as early as 4.0 to 4.2 Ga, whereas if it occurred at the surface of the earth abiogenesis could only have occurred between 3.7 and 4.0 Ga.<ref>{{cite journal |last=Maher |first=Kevin A. |authorlink= |coauthors=Stephenson, David J. |year=1988 |month= |title=Impact frustration of the origin of life |journal=Nature |volume=331 |issue=6157 |pages=612–4 |doi=10.1038/331612a0 }}</ref> | |||
| {{Main|Panspermia}} | |||
| Another ancient idea dating back to ] in the 5th century BC is ],<ref name="Gerda Horneck">{{cite journal |last1=Horneck |first1=Gerda |last2=Klaus |first2=David M. |last3=Mancinelli |first3=Rocco L. |date=March 2010 |title=Space Microbiology |journal=] |volume=74 |issue=1 |pages=121–156 |doi=10.1128/MMBR.00016-09 |pmc=2832349 |pmid=20197502 |bibcode=2010MMBR...74..121H}}</ref> the idea that ] originated elsewhere in the ] and came to Earth. The modern version of panspermia holds that life may have been distributed to Earth by ], ], ]<ref name="cometary panspermia">{{cite journal |last=Wickramasinghe |first=Chandra |author-link=Chandra Wickramasinghe |title=Bacterial morphologies supporting cometary panspermia: a reappraisal |journal=] |year=2011 |volume=10 |issue=1 |pages=25–30 |doi=10.1017/S1473550410000157 |bibcode=2011IJAsB..10...25W |citeseerx=10.1.1.368.4449 |s2cid=7262449}}</ref> and ].<ref>Rampelotto, P. H. (2010). "Panspermia: A promising field of research". In: Astrobiology Science Conference. Abs 5224.</ref> It does not attempt to explain how life originated in itself, but shifts the origin of life on Earth to another heavenly body. The advantage is that life is not required to have formed on each planet it occurs on, but rather in a more limited set of locations, or even a single location, and then spread about the ] to other star systems via cometary or meteorite impact.<ref name="NYT-20160912">{{cite news |last=Chang |first=Kenneth |title=Visions of Life on Mars in Earth's Depths |url=https://www.nytimes.com/2016/09/13/science/south-african-mine-life-on-mars.html |date=12 September 2016 |work=] |access-date=12 September 2016 |url-status=live |archive-url=https://web.archive.org/web/20160912225220/http://www.nytimes.com/2016/09/13/science/south-african-mine-life-on-mars.html |archive-date=12 September 2016}}</ref> Panspermia did not get much scientific support because it was largely used to deflect the need of an answer instead of explaining observable phenomena. Although the interest in panspermia grew when the study of meteorites found traces of organic materials in them, it is currently accepted that life started locally on Earth.<ref>{{cite book |last= Aguilera Mochón|first= Juan Antonio|date= 2016|title= El origen de la vida en la tierra|trans-title= The origin of life on Earth|url= |language= Spanish|location= Spain|publisher= RBA|isbn=978-84-473-8386-3}}</ref> | |||
| === "A warm little pond": primordial soup === | |||
| Other research suggests a colder start to life. Work by ] and colleagues on the synthesis of purines has shown that freezing temperatures are advantageous, due to the concentrating effect for key precursors such as ].<ref>{{cite journal |last=Orgel |first=Leslie E. |year=2004 |title=Prebiotic adenine revisited: Eutectics and photochemistry|journal=Origins of Life and Evolution of Biospheres |volume=34 |pages=361–9| doi=10.1023/B:ORIG.0000029882.52156.c2 }}</ref> | |||
| Research by ] and colleagues suggested that while adenine and guanine require freezing conditions for synthesis, cytosine and uracil may require boiling temperatures.<ref>{{cite journal |last=Robertson |first=Michael P. |authorlink= |coauthors=Miller, Stanley L. |year=1995 |month= |title=An efficient prebiotic synthesis of cytosine and uracil |journal=Nature |volume=375 |issue=6534 |pages=772–774 |doi=10.1038/375772a0 }}</ref> Based on this research, Miller suggested a beginning of life involving freezing conditions and exploding meteorites.<ref>{{cite journal |last=Bada |first=J. L. |authorlink= |coauthors=Bigham, C.; Miller, S. L. |year=1994 |month= |title=Impact Melting of Frozen Oceans on the Early Earth: Implications for the Origin of Life |journal=] |volume=91 |issue=4 |pages=1248–50 |url=http://www.pnas.org/cgi/content/abstract/91/4/1248 |doi=10.1073/pnas.91.4.1248|format=abstract|pmid=11539550 }}</ref> A new article in ] points to research by the Miller group indicating the formation of seven different amino acids and 11 types of nucleobases in ice when ] and ] were left in a freezer from 1972–1997.<ref>{{cite web |url=http://discovermagazine.com/2008/feb/did-life-evolve-in-ice/article_view?b_start:int=0&-C= |title=Did Life Evolve in Ice? | Arctic & Antarctic | publisher=DISCOVER Magazine |format= |work= |accessdate=2008-07-03}}</ref><ref>{{cite journal |last=Levy |first=M. |coauthors=Miller, S. L.; Brinton, K.; Bada, J. L. |year=2000 |month=June |title=Prebiotic synthesis of adenine and amino acids under Europa-like conditions |journal=Icarus |volume=145 |issue=2 |pages=609–13 |pmid=11543508 |accessdate= 2008-02-11 |doi=10.1006/icar.2000.6365 <!--Retrieved from Yahoo! by DOI bot-->}}</ref> This article also describes research by Hauke Trinks showing the formation of RNA molecules 400 bases long under freezing conditions using an RNA template, a single-strand chain of RNA that guides the formation of a new strand of RNA. As that new RNA strand grows, it adheres to the template.<ref>{{cite journal |last=Trinks |first=Hauke |coauthors=Schröder, Wolfgang; Biebricher, Christof | |||
| |year=2005 |month=October |title=Ice And The Origin Of Life |journal=Origins of Life and Evolution of the Biosphere |volume=35 |issue=5 |pages=429–45 |doi=10.1007/s11084-005-5009-1 |url=http://www.ingentaconnect.com/content/klu/orig/2005/00000035/00000005/00005009#aff_1 |accessdate= 2008-02-11 }}</ref> The explanation given for the unusual speed of these reactions at such a low temperature is ]. As an ice crystal forms, it stays pure: only molecules of water join the growing crystal, while impurities like salt or cyanide are excluded. These impurities become crowded in microscopic pockets of liquid within the ice, and this crowding causes the molecules to collide more often. | |||
| {{Main|Primordial soup}} | |||
| Evidence of the early appearance of life comes from the ] supercrustal belt in Western Greenland and from similar formations in the nearby ]s. Carbon entering into rock formations has a ratio of ] (<sup>13</sup>C) to ] (<sup>12</sup>C) of about −5.5 (in units of ]), where because of a preferential biotic uptake of <sup>12</sup>C, ] has a δ<sup>13</sup>C of between −20 and −30. These isotopic fingerprints are preserved in the sediments, and Mojzis has used this technique to suggest that life existed on the planet already by 3.85 billion years ago.<ref name="Mojzis">{{cite journal |last=Mojzis |first=S. J. |authorlink= |coauthors=''et al.'' |year=1996 |month= |title=Evidence for life on earth before 3,800 million years ago |journal=Nature |volume=384 |issue=6604 |pages=55–9 |doi=10.1038/384055a0 }}</ref> Lazcano and Miller (1994) suggest that the rapidity of the evolution of life is dictated by the rate of recirculating water through mid-ocean submarine vents. Complete recirculation takes 10 million years, thus any organic compounds produced by then would be altered or destroyed by temperatures exceeding {{convert|300|°C|°F}}. They estimate that the development of a 100 kilobase genome of a DNA/protein primitive ] into a 7000 gene filamentous ] would have required only 7 Ma.<ref>{{cite journal |last=Lazcano |first=A. |authorlink= |coauthors=Miller, S. L. |year=1994 |month= |title=How long did it take for life to begin and evolve to cyanobacteria? |journal=Journal of Molecular Evolution |volume=39 |issue= |pages=546–54 |doi=10.1007/BF00160399 }}</ref> | |||
| The idea that life originated from non-living matter in slow stages appeared in ]'s 1864–1867 book ''Principles of Biology'', and in ]'s 1879 paper "On spontaneous generation and evolution". On 1 February 1871 ] wrote about these publications to ], and set out his own speculation, suggesting that the original spark of life may have begun in a "warm little pond, with all sorts of ] and phosphoric ], light, heat, electricity, {{sic|hide=y|&c.}}, present, that a {{sic|hide=y|proteine}} compound was chemically formed ready to undergo still more complex changes." Darwin went on to explain that "at the present day such matter would be instantly devoured or absorbed, which would not have been the case before living creatures were formed."<ref name="Darwin DCP-LETT-7471">{{cite web |title=Letter no. 7471, Charles Darwin to Joseph Dalton Hooker, 1 February (1871) |website=Darwin Correspondence Project |url=https://www.darwinproject.ac.uk/letter/DCP-LETT-7471.xml |access-date=7 July 2020 |archive-date=7 July 2020 |archive-url=https://web.archive.org/web/20200707094423/https://www.darwinproject.ac.uk/letter/DCP-LETT-7471.xml |url-status=live }}</ref><ref>{{cite web |title=Origin and Evolution of Life on a Frozen Earth |last=Priscu |first=John C. |author-link=John Charles Priscu |publisher=] |location=Arlington County, Virginia |url=https://www.nsf.gov/news/special_reports/darwin/textonly/polar_essay1.jsp |access-date=1 March 2014 |url-status=live |archive-url=https://web.archive.org/web/20131218070241/http://www.nsf.gov/news/special_reports/darwin/textonly/polar_essay1.jsp |archive-date=18 December 2013}}</ref><ref name="BBC-20201111">{{cite news |last=Marshall |first=Michael |title=Charles Darwin's hunch about early life was probably right |url=https://www.bbc.com/future/article/20201110-charles-darwin-early-life-theory |date=11 November 2020 |work=] |access-date=11 November 2020 |archive-date=11 November 2020 |archive-url=https://web.archive.org/web/20201111015900/https://www.bbc.com/future/article/20201110-charles-darwin-early-life-theory |url-status=live }}</ref> | |||
| ==Current models== | |||
| There is no truly "standard model" of the origin of life. Most currently accepted models draw at least some elements from the framework laid out by the Oparin-Haldane hypothesis. Under that umbrella, however, are a wide array of disparate discoveries and conjectures such as the following, listed in a rough order of postulated emergence: | |||
| # Some theorists suggest that the atmosphere of the early Earth may have been ] in nature, composed primary of ] (CH<sub>4</sub>), ] (NH<sub>3</sub>), ] (H<sub>2</sub>O), ] (H<sub>2</sub>S), ] (CO<sub>2</sub>) or ] (CO), and ] (PO<sub>4</sub><sup>3-</sup>), with molecular ] (O<sub>2</sub>) and ] (O<sub>3</sub>) either rare or absent. | |||
| # In such a reducing atmosphere, electrical activity can catalyze the creation of certain basic small ]s (]s) of life, such as amino acids. This was demonstrated in the ] by ] and ] in 1953. | |||
| # ]s (of an appropriate length) can spontaneously form ]s, a basic component of the ]. | |||
| # A fundamental question is about the nature of the first self-replicating molecule. Since replication is accomplished in modern cells through the cooperative action of proteins and nucleic acids, the major schools of thought about how the process originated can be broadly classified as "proteins first" and "nucleic acids first". | |||
| # The principal thrust of the "nucleic acids first" argument is as follows: | |||
| ## The ]ization of ]s into random ] molecules might have resulted in self-replicating ]s (]) | |||
| ## ] pressures for catalytic efficiency and diversity might have resulted in ribozymes which catalyse ] (hence formation of small proteins), since oligopeptides complex with RNA to form better catalysts. The first ] might have been created by such a process, resulting in more prevalent protein synthesis. | |||
| ## Synthesized ] might then outcompete ribozymes in catalytic ability, and therefore become the dominant biopolymer, relegating nucleic acids to their modern use, predominantly as a carrier of ] information. | |||
| ] in 1924 and ] in 1929 proposed that the first molecules constituting the earliest cells slowly self-organized from a ], and this theory is called the ''']'''.<ref name="Bahadur1973">{{cite journal |last=Bahadur |first=Krishna |year=1973 |title=Photochemical Formation of Self–sustaining Coacervates |journal=] |volume=39 |issue=4 |pages=455–467 |doi=10.1016/S0044-4057(75)80076-1 |pmid=1242552 |url=http://www.dli.gov.in/rawdataupload/upload/insa/INSA_1/20005b73_455.pdf |archive-url=https://web.archive.org/web/20131019172800/http://www.dli.gov.in/rawdataupload/upload/insa/INSA_1/20005b73_455.pdf |archive-date=19 October 2013}}</ref><ref name="Bahadur1975">{{cite journal |last=Bahadur |first=Krishna |year=1975 |title=Photochemical Formation of Self-Sustaining Coacervates |journal=] |url=https://www.sciencedirect.com/science/article/abs/pii/S0044405775800761 |volume=130 |issue=3 |pages=211–218 |doi=10.1016/S0044-4057(75)80076-1 |oclc=641018092 |pmid=1242552 |access-date=13 December 2022 |archive-date=13 December 2022 |archive-url=https://web.archive.org/web/20221213115635/https://www.sciencedirect.com/science/article/abs/pii/S0044405775800761 |url-status=live }}</ref> Haldane suggested that the Earth's prebiotic oceans consisted of a "hot dilute soup" in which organic compounds could have formed.<ref name="Bernal 1967"/><ref>{{harvnb|Bryson|2004|pp=300–302}}</ref> ] showed that such mechanisms could form most of the necessary molecules for life from inorganic precursors.<ref>{{harvnb|Bernal|1951}}</ref> In 1967, he suggested three "stages": the origin of biological ]s; the origin of biological ]s; and the evolution from molecules to cells.<ref>{{cite journal |last=Martin |first=William F. |author-link=William F. Martin |date=January 2003 |title=On the origins of cells: a hypothesis for the evolutionary transitions from abiotic geochemistry to chemoautotrophic prokaryotes, and from prokaryotes to nucleated cells |journal=Phil. Trans. R. Soc. Lond. A |volume=358 |issue=1429 |pages=59–83 |doi=10.1098/rstb.2002.1183 |pmid=12594918 |pmc=1693102}}</ref><ref>{{cite journal |last=Bernal |first=John Desmond |author-link=John Desmond Bernal |date=September 1949 |title=The Physical Basis of Life |journal=] |volume=62 |issue=9 |pages=537–558 |bibcode=1949PPSA...62..537B |doi=10.1088/0370-1298/62/9/301 |s2cid=83754271}}</ref> | |||
| As of 2009, no one has yet synthesized a "protocell" using basic components which would have the necessary properties of life (the so-called ''"bottom-up-approach"''). Without such a proof-of-principle, explanations have tended to be short on specifics. However, some researchers are working in this field, notably ] at ] and ] at ]. Others have argued that a ''"top-down approach"'' is more feasible. One such approach, attempted by ] and others at ], involves engineering existing prokaryotic cells with progressively fewer genes, attempting to discern at which point the most minimal requirements for life were reached. The biologist ], coined the term Biopoesis for this process, and suggested that there were a number of clearly defined "stages" that could be recognised in explaining the origin of life. | |||
| * Stage 1: The origin of biological ] | |||
| * Stage 2: The origin of biological ] | |||
| * Stage 3: The evolution from molecules to cell | |||
| === Miller–Urey experiment === | |||
| Bernal suggested that ] may have commenced early, some time between Stage 1 and 2. | |||
| {{Main|Miller–Urey experiment}} | |||
| ===Origin of organic molecules=== | |||
| There are two possible sources of organic molecules on the early Earth: | |||
| # Terrestrial origins - organic synthesis driven by impact shocks or by other energy sources (such as ultraviolet light or electrical discharges) (eg.Miller's experiments) | |||
| # Extraterrestrial origins - delivery by objects (eg carbonaceous ]) or gravitational attraction of organic molecules or primitive life-forms from space | |||
| In 1952, ] and ] carried out a chemical experiment to demonstrate how organic molecules could have formed spontaneously from inorganic precursors under ] like those posited by the Oparin–Haldane hypothesis. It used a highly ] (lacking oxygen) mixture of gases—], ], and ], as well as ]—to form simple organic monomers such as ]s.<ref>{{cite journal |last=Miller |first=Stanley L. |author-link=Stanley Miller |date=15 May 1953 |title=A Production of Amino Acids Under Possible Primitive Earth Conditions |journal=] |volume=117 |issue=3046 |pages=528–529 |bibcode=1953Sci...117..528M |doi=10.1126/science.117.3046.528 |pmid=13056598}}</ref><ref name="pmid21422282">{{cite journal |last1=Parker |first1=Eric T. |last2=Cleaves |first2=Henderson J. |last3=Dworkin |first3=Jason P. |last4=Glavin |first4=Daniel P. |last5=Callahan |first5=Michael |last6=Aubrey |first6=Andrew |last7=Lazcano |first7=Antonio |author7-link=Antonio Lazcano |last8=Bada |first8=Jeffrey L. |author8-link=Jeffrey L. Bada |display-authors=3 |date=5 April 2011 |title=Primordial synthesis of amines and amino acids in a 1958 Miller H<sub>2</sub>S-rich spark discharge experiment |journal=] |volume=108 |issue=14 |pages=5526–5531 |bibcode=2011PNAS..108.5526P |doi=10.1073/pnas.1019191108 |pmc=3078417 |pmid=21422282 |doi-access=free}}</ref> Bernal said of the Miller–Urey experiment that "it is not enough to explain the formation of such molecules, what is necessary, is a physical-chemical explanation of the origins of these molecules that suggests the presence of suitable sources and sinks for free energy."<ref>{{harvnb|Bernal|1967|p=143}}</ref> However, current scientific consensus describes the primitive atmosphere as weakly reducing or neutral,<ref name="Cleaves 2008">{{cite journal |last1=Cleaves |first1=H. James |last2=Chalmers |first2=John H. |last3=Lazcano |first3=Antonio |author3-link=Antonio Lazcano |last4=Miller |first4=Stanley L. |last5=Bada |first5=Jeffrey L. |author5-link=Jeffrey L. Bada |display-authors=3 |date=April 2008 |title=A Reassessment of Prebiotic Organic Synthesis in Neutral Planetary Atmospheres |journal=] |volume=38 |issue=2 |pages=105–115 |bibcode=2008OLEB...38..105C |doi=10.1007/s11084-007-9120-3 |pmid=18204914 |s2cid=7731172}}</ref><ref name="Chyba 2005">{{cite journal |last=Chyba |first=Christopher F. |author-link=Christopher Chyba |s2cid=93303848 |date=13 May 2005 |title=Rethinking Earth's Early Atmosphere |journal=] |volume=308 |issue=5724 |pages=962–963 |doi=10.1126/science.1113157 |pmid=15890865}}</ref> diminishing the amount and variety of amino acids that could be produced. The addition of ] and ] minerals, present in early oceans, however, produces a diverse array of amino acids.<ref name="Cleaves 2008"/> Later work has focused on two other potential reducing environments: ] and deep-sea hydrothermal vents.<ref>{{harvnb|Barton|Briggs|Eisen|Goldstein|2007|pp=93–95}}</ref><ref>{{harvnb|Bada|Lazcano|2009|pp=56–57}}</ref><ref name="Bada 2003">{{cite journal |last1=Bada |first1=Jeffrey L. |author1-link=Jeffrey L. Bada |last2=Lazcano |first2=Antonio |author2-link=Antonio Lazcano |date=2 May 2003 |url=http://astrobiology.berkeley.edu/PDFs_articles/Bada_Science2003.pdf |title=Prebiotic Soup – Revisiting the Miller Experiment |journal=] |volume=300 |issue=5620 |pages=745–746 |doi=10.1126/science.1085145 |pmid=12730584 |s2cid=93020326 |access-date=2015-06-13 |url-status=live |archive-url=https://web.archive.org/web/20160304222002/http://astrobiology.berkeley.edu/PDFs_articles/Bada_Science2003.pdf |archive-date=4 March 2016}}</ref> | |||
| Recently, estimates of these sources suggest that the heavy bombardment before 3.5 Ga within the early atmosphere made available quantities of organics comparable to those produced by other energy sources.<ref>{{cite journal |last=Chyba |first=Christopher |authorlink= |coauthors=Sagan, Carl |year=1992 |month= |title=Endogenous production, exogenous delivery and impact-shock synthesis of organic molecules: an inventory for the origins of life |journal=Nature |volume=355 |issue=6356 |pages=125–32 |doi=10.1038/355125a0 }}</ref> | |||
| <!-- Please do not extend this section; it is a summary of the linked "main" article. Put your materials there, and THEN if absolutely necessary adjust this summary to match, very briefly. --> | |||
| == Producing a habitable Earth == | |||
| ===="Soup" theory today: Miller's experiment and subsequent work==== | |||
| Biochemist Robert Shapiro has summarized the "Primordial Soup" theory of Oparin and Haldane in its "mature form" as follows:<ref>{{cite book |last=Shapiro |first=Robert | title=Origins: A Skeptic's Guide to the Creation of Life on Earth | publisher=Bantam Books | year=1987 | page=110 }}</ref> | |||
| # The early Earth had a chemically reducing atmosphere, as discussed above. | |||
| # This atmosphere, exposed to energy in various forms, produced simple organic compounds ("]s"). | |||
| # These compounds accumulated in a "soup". | |||
| # By further transformation, more complex organic ]s— and ultimately life— developed in the soup. | |||
| {{Abiogenesis timeline}} | |||
| =====Regarding the reducing atmosphere===== | |||
| Whether the mixture of gases used in the Miller-Urey experiment truly reflects the atmospheric content of ] is a controversial topic. Other less reducing gases produce a lower yield and variety. It was once thought that appreciable amounts of molecular oxygen were present in the prebiotic atmosphere, which would have essentially prevented the formation of organic molecules; however, the current scientific consensus is that such was not the case. (See ]). | |||
| === Evolutionary history === | |||
| =====Regarding monomer formation===== | |||
| {{Main|Miller experiment}} | |||
| One of the most important pieces of experimental support for the "soup" theory came in 1953. A graduate student, ], and his professor, ], performed an experiment that demonstrated how organic molecules could have spontaneously formed from inorganic precursors, under conditions like those posited by the Oparin-Haldane Hypothesis. The now-famous "]" used a highly reduced mixture of gases–], ammonia and ]–to form basic organic ]s, such as amino acids.<ref>{{cite journal |last=Miller |first=Stanley L. |authorlink= |coauthors= |year=1953 |title=A Production of Amino Acids Under Possible Primitive Earth Conditions|journal=Science |volume=117 |pages=528–9 |doi=10.1126/science.117.3046.528 |pmid=13056598 }}</ref> This provided direct experimental support for the second point of the "soup" theory as described above, and it is around the remaining three points of the theory that much of the debate now centers. | |||
| ==== Early universe with first stars ==== | |||
| Apart from the Miller-Urey experiment, described above, the next most important step in research on prebiotic organic synthesis was the demonstration by John Oró that the nucleic acid purine base, adenine, was formed by heating aqueous ammonium cyanide solutions.<ref>{{cite journal |last=Oró |first=J. |year=1961 |title=Mechanism of synthesis of adenine from hydrogen cyanide under possible primitive Earth conditions|journal=Nature |volume=191 |pages=1193–4 |doi=10.1038/1911193a0 }}</ref> In support of abiogenisis in eutectic ice (see ]), more recent work demonstrated the formation of s-]s (alternative ]s), ]s (including cytosine and uracil), and adenine from urea solutions subjected to freeze-thaw cycles under a reductive atmosphere (with spark discharges as an energy source).<ref>{{cite journal | author = Menor-Salván C, Ruiz-Bermejo DM, Guzmán MI, Osuna-Esteban S, Veintemillas-Verdaguer S | title = Synthesis of pyrimidines and triazines in ice: implications for the prebiotic chemistry of nucleobases. | journal = Chemistry | year = 2007 | volume = 15 (17) | pages = 4411-8 |pmc=19288488 | doi = 10.1002/chem.200802656 | pmid = 19288488 }}</ref> | |||
| {{See also|Chronology of the universe}} | |||
| =====Regarding monomer accumulation===== | |||
| The "soup" theory relies on the assumption proposed by Darwin (see above) that in an environment with no pre-existing life, organic molecules may have accumulated and provided an environment for ]. | |||
| Soon after the ], which occurred roughly 14 Gya, the only chemical elements present in the universe were ], ], and ], the three lightest atoms in the periodic table. These elements gradually accreted and began orbiting in disks of gas and dust. Gravitational accretion of material at the hot and dense centers of these ]s formed stars by the fusion of hydrogen.<ref>{{Cite journal |last1=Madau |first1=Piero |last2=Dickinson |first2=Mark |date=2014-08-18 |title=Cosmic Star-Formation History |url=https://www.annualreviews.org/doi/10.1146/annurev-astro-081811-125615 |journal=Annual Review of Astronomy and Astrophysics |volume=52 |issue=1 |pages=415–486 |doi=10.1146/annurev-astro-081811-125615 |arxiv=1403.0007 |bibcode=2014ARA&A..52..415M |s2cid=658354 |access-date=8 December 2023 |archive-date=1 July 2022 |archive-url=https://web.archive.org/web/20220701214618/https://www.annualreviews.org/doi/10.1146/annurev-astro-081811-125615 |url-status=live }}</ref> Early stars were massive and short-lived, producing all the heavier elements through ]. Element formation through stellar nucleosynthesis proceeds to its most stable element Iron-]. Heavier elements were formed during supernovae at the end of a stars lifecycle. ], currently the ] in the universe (after hydrogen, helium, and ]), was formed mainly in ], particularly those bigger than twice the mass of the sun.<ref name="NA-20200706">{{cite journal |last=Marigo |first=Paola |display-authors=|date=6 July 2020 |title=Carbon star formation as seen through the non-monotonic initial–final mass relation |url=https://www.nature.com/articles/s41550-020-1132-1 |url-status=live |journal=] |volume=152 |issue=11 |pages=1102–1110 |arxiv=2007.04163 |bibcode=2020NatAs...4.1102M |doi=10.1038/s41550-020-1132-1 |s2cid=220403402 |archive-url=https://web.archive.org/web/20230216160258/https://www.nature.com/articles/s41550-020-1132-1 |archive-date=16 February 2023 |access-date=7 July 2020}}</ref> As these stars reached the end of their ], they ejected these heavier elements, among them carbon and oxygen, throughout the universe. These heavier elements allowed for the formation of new objects, including rocky planets and other bodies.<ref>{{cite web |title=WMAP- Life in the Universe |url=https://wmap.gsfc.nasa.gov/universe/uni_life.html |url-status=live |archive-url=https://web.archive.org/web/20230129215644/https://wmap.gsfc.nasa.gov/universe/uni_life.html |archive-date=29 January 2023 |access-date=27 September 2019}}</ref> According to the ], the formation and evolution of the ] began 4.6 Gya with the ] of a small part of a giant ]. Most of the collapsing mass collected in the center, forming the ], while the rest flattened into a ] out of which the ]s, ], ]s, and other small Solar System bodies formed.<ref>{{cite web |title=Formation of Solar Systems: Solar Nebular Theory |url=http://www.astro.umass.edu/~myun/teaching/a100_old/solarnebulartheory.htm |url-status=live |archive-url=https://web.archive.org/web/20190927152503/http://www.astro.umass.edu/~myun/teaching/a100_old/solarnebulartheory.htm |archive-date=27 September 2019 |access-date=27 September 2019 |publisher=]}}</ref> | |||
| =====Regarding further transformation===== | |||
| The spontaneous formation of complex ]s from abiotically generated monomers under the conditions posited by the "soup" theory is not at all a straightforward process. Besides the necessary basic organic monomers, compounds that would have prohibited the formation of polymers were formed in high concentration during the Miller-Urey and Oró experiments. The Miller experiment, for example, produces many substances that would undergo cross-reactions with the amino acids or terminate the peptide chain. | |||
| ==== Emergence of Earth ==== | |||
| More fundamentally, it can be argued that the most crucial challenge unanswered by this theory is how the relatively simple organic building blocks polymerise and form more complex structures, interacting in consistent ways to form a protocell. For example, in an aqueous environment ] of oligomers/polymers into their constituent monomers would be favored over the condensation of individual monomers into polymers. | |||
| {{See also|Geological history of Earth|Circumstellar habitable zone|Prebiotic atmosphere}} | |||
| ====The deep sea vent theory==== | |||
| The deep sea vent, or ], theory for the origin of life on Earth posits that life may have begun at submarine hydrothermal vents, where hydrogen-rich fluids emerge from below the sea floor and interface with carbon dioxide-rich ocean water. Sustained chemical energy in such systems is derived from ]s, in which electron donors, such as molecular hydrogen, react with electron acceptors, such as carbon dioxide (see ]). | |||
| The age of the ] is 4.54 Gya as found by radiometric dating of ] in ] meteorites, the oldest material in the Solar System.<ref name="USGS1997">{{cite web |date=9 July 2007 |title=Age of the Earth |url=https://pubs.usgs.gov/gip/geotime/age.html |url-status=live |archive-url=https://web.archive.org/web/20051223072700/http://pubs.usgs.gov/gip/geotime/age.html |archive-date=23 December 2005 |access-date=10 January 2006 |publisher=]}}</ref><ref>{{harvnb|Dalrymple|2001|pp=205–221}}</ref> Earth, during the ] eon (from its formation until 4.031 Gya,) was at first inhospitable to any living organisms. During its formation, the Earth lost a significant part of its initial mass, and consequentially lacked the ] to hold molecular hydrogen and the bulk of the original inert gases.<ref>{{harvnb|Fesenkov|1959|p=9}}</ref> Soon after initial accretion of Earth at 4.48 Ga, its collision with ], a hypothesised impactor, is thought to have created the ejected debris that would eventually form the Moon.<ref>{{Cite journal |last1=Bottke |first1=W. F. |last2=Vokrouhlický |first2=D. |last3=Marchi |first3=S. |last4=Swindle |first4=T. |last5=Scott |first5=E. R. D. |last6=Weirich |first6=J. R. |last7=Levison |first7=H. |date=2015-04-17 |title=Dating the Moon-forming impact event with asteroidal meteorites |journal=Science |volume=348 |issue=6232 |pages=321–323 |doi=10.1126/science.aaa0602 |bibcode=2015Sci...348..321B |s2cid=206632612 |doi-access=free |pmid=25883354 }}</ref> This impact would have removed the Earth's primary atmosphere, leaving behind clouds of viscous silicates and carbon dioxide. This unstable atmosphere was short-lived and condensed shortly after to form the bulk silicate Earth, leaving behind an atmosphere largely consisting of water vapor, ], and ], with smaller amounts of ], hydrogen, and ] compounds.<ref>{{cite journal |last=Kasting |first=James F. |author-link=James Kasting |date=12 February 1993 |title=Earth's Early Atmosphere |url=http://wwwdca.iag.usp.br/www/material/fornaro/ACA410/Kasting%201993_EarthEarlyAtmos.pdf |journal=] |volume=259 |issue=5097 |pages=920–926 |bibcode=1993Sci...259..920K |doi=10.1126/science.11536547 |pmid=11536547 |s2cid=21134564 |archive-url=https://web.archive.org/web/20151010074651/http://wwwdca.iag.usp.br/www/material/fornaro/ACA410/Kasting%201993_EarthEarlyAtmos.pdf |archive-date=10 October 2015 |access-date=2015-07-28}}</ref><ref name="Follmann2009">{{cite journal |last1=Follmann |first1=Hartmut |last2=Brownson |first2=Carol |date=November 2009 |title=Darwin's warm little pond revisited: from molecules to the origin of life |journal=] |volume=96 |issue=11 |pages=1265–1292 |bibcode=2009NW.....96.1265F |doi=10.1007/s00114-009-0602-1 |pmid=19760276 |s2cid=23259886}}</ref> The solution of carbon dioxide in water is thought to have made the seas slightly ]ic, with a ] of about 5.5.<ref>{{cite journal |last=Morse |first=John |date=September 1998 |title=Hadean Ocean Carbonate Geochemistry |journal=Aquatic Geochemistry |volume=4 |issue=3/4 |pages=301–319 |bibcode=1998MinM...62.1027M |doi=10.1023/A:1009632230875 |s2cid=129616933}}</ref> | |||
| ====Fox's experiments==== | |||
| In the 1950s and 1960s, ] studied the spontaneous formation of ] structures under conditions that might plausibly have existed early in Earth's history. He demonstrated that amino acids could spontaneously form small peptides. These amino acids and small peptides could be encouraged to form closed spherical membranes, called ].<ref name="foxexp"> Nitro.biosci.arizona.edu, Retrieved on ]</ref> | |||
| Condensation to form liquid ] is theorised to have occurred as early as the Moon-forming impact.<ref>{{Cite journal |last1=Sleep |first1=Norman H. |last2=Zahnle |first2=Kevin J. |last3=Lupu |first3=Roxana E. |date=2014-09-13 |title=Terrestrial aftermath of the Moon-forming impact |journal=Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences |volume=372 |issue=2024 |pages=20130172 |doi=10.1098/rsta.2013.0172 |pmid=25114303 |bibcode=2014RSPTA.37230172S |s2cid=6902632 |doi-access=free }}</ref><ref>{{Cite journal |last1=Morse |first1=John W. |last2=Mackenzie |first2=Fred T. |date=1998 |title= |url=http://link.springer.com/10.1023/A:1009632230875 |journal=Aquatic Geochemistry |volume=4 |issue=3/4 |pages=301–319 |doi=10.1023/A:1009632230875 |s2cid=129616933 |access-date=8 December 2023 |archive-date=31 January 2024 |archive-url=https://web.archive.org/web/20240131154932/https://link.springer.com/article/10.1023/A:1009632230875 |url-status=live }}</ref> This scenario has found support from the dating of 4.404 Gya ] crystals with high ] values from metamorphosed ] of ] in Western Australia.<ref>{{Cite journal |last1=Crowley |first1=James L. |last2=Myers |first2=John S. |last3=Sylvester |first3=Paul J |last4=Cox |first4=Richard A. |date=May 2005 |title=Detrital Zircon from the Jack Hills and Mount Narryer, Western Australia: Evidence for Diverse >4.0 Ga Source Rocks |url=https://www.journals.uchicago.edu/doi/10.1086/428804 |journal=The Journal of Geology |volume=113 |issue=3 |pages=239–263 |doi=10.1086/428804 |bibcode=2005JG....113..239C |s2cid=140715676 |access-date=8 December 2023 |archive-date=16 December 2023 |archive-url=https://web.archive.org/web/20231216004103/https://www.journals.uchicago.edu/doi/10.1086/428804 |url-status=live }}</ref><ref name="Wilde 2001">{{cite journal |last1=Wilde |first1=Simon A. |last2=Valley |first2=John W. |last3=Peck |first3=William H. |last4=Graham |first4=Colin M. |date=11 January 2001 |title=Evidence from detrital zircons for the existence of continental crust and oceans on the Earth 4.4 Gyr ago |url=http://www.geology.wisc.edu/~valley/zircons/Wilde2001Nature.pdf |url-status=live |journal=] |volume=409 |issue=6817 |pages=175–178 |bibcode=2001Natur.409..175W |doi=10.1038/35051550 |pmid=11196637 |s2cid=4319774 |archive-url=https://web.archive.org/web/20150605132344/http://www.geology.wisc.edu/~valley/zircons/Wilde2001Nature.pdf |archive-date=5 June 2015 |access-date=3 June 2015}}</ref> The Hadean atmosphere has been characterized as a "gigantic, productive outdoor chemical laboratory," similar to volcanic gases today which still support some abiotic chemistry. Despite the likely increased volcanism from early plate tectonics, the Earth may have been a predominantly water world between 4.4 and 4.3 Gya. It is debated whether or not crust was exposed above this ocean due to uncertainties of what early plate tectonics looked like. For early life to have developed, it is generally thought that a land setting is required, so this question is essential to determining when in Earth's history life evolved.<ref>{{Cite journal |last=Korenaga |first=Jun |date=December 2008 |title=Plate tectonics, flood basalts and the evolution of Earth's oceans |journal=Terra Nova |volume=20 |issue=6 |pages=419–439 |doi=10.1111/j.1365-3121.2008.00843.x |bibcode=2008TeNov..20..419K |s2cid=36766331 |doi-access=free }}</ref> Immediately after the Moon-forming impact, Earth likely had little if any continental crust, a turbulent atmosphere, and a ] subject to intense ] light from a ]. It was also affected by ], and continued asteroid and ] impacts.<ref name="rise.2006">{{cite journal |last1=Rosing |first1=Minik T. |last2=Bird |first2=Dennis K. |last3=Sleep |first3=Norman H. |last4=Glassley |first4=William |last5=Albarède |first5=Francis |author-link5=Francis Albarède |display-authors=3 |date=22 March 2006 |title=The rise of continents – An essay on the geologic consequences of photosynthesis |url=https://www.researchgate.net/publication/223066196 |url-status=live |journal=] |volume=232 |issue=2–4 |pages=99–113 |bibcode=2006PPP...232...99R |doi=10.1016/j.palaeo.2006.01.007 |archive-url=https://web.archive.org/web/20150714073656/http://www.researchgate.net/profile/Francis_Albarede/publication/223066196_The_rise_of_continentsAn_essay_on_the_geologic_consequences_of_photosynthesis/links/00b7d51766c442f58b000000.pdf |archive-date=14 July 2015 |access-date=2015-06-08}}</ref> Despite all this, niche environments likely existed conducive to life on Earth in the Late-Hadean to Early-Archaean. | |||
| ====Eigen's hypothesis==== | |||
| In the early 1970s the problem of the origin of life was approached by ] and ] of the ]. They examined the transient stages between the molecular chaos and a self-replicating ] in a prebiotic soup.<ref>{{cite book |author=Schuster, P.; Eigen, M. |title=The hypercycle, a principle of natural self-organization |publisher=Springer-Verlag |location=Berlin |year=1979 |isbn=0-387-09293-5 }}</ref> | |||
| The ] hypothesis posits that a period of intense impact occurred at 4.1 to 3.8 Gya during the Hadean and early ] eons.<ref>{{Cite journal |last1=Tera |first1=Fouad |last2=Papanastassiou |first2=D.A. |last3=Wasserburg |first3=G.J. |date=April 1974 |title=Isotopic evidence for a terminal lunar cataclysm |journal=Earth and Planetary Science Letters |volume=22 |issue=1 |pages=1–21 |doi=10.1016/0012-821x(74)90059-4 |bibcode=1974E&PSL..22....1T |url=https://www.sciencedirect.com/science/article/abs/pii/0012821X74900594 |archive-date=31 January 2024 |archive-url=https://web.archive.org/web/20240131154923/https://www.sciencedirect.com/science/article/abs/pii/0012821X74900594?via%3Dihub |url-status=live }}</ref><ref>{{Cite journal |last=Stoffler |first=D. |date=2006-01-01 |title=Cratering History and Lunar Chronology |journal=Reviews in Mineralogy and Geochemistry |volume=60 |issue=1 |pages=519–596 |doi=10.2138/rmg.2006.60.05 |bibcode=2006RvMG...60..519S |url=https://pubs.geoscienceworld.org/msa/rimg/article-abstract/60/1/519/140783/Cratering-History-and-Lunar-Chronology?redirectedFrom=fulltext |archive-date=31 January 2024 |archive-url=https://web.archive.org/web/20240131154819/https://pubs.geoscienceworld.org/msa/rimg/article-abstract/60/1/519/140783/Cratering-History-and-Lunar-Chronology?redirectedFrom=fulltext |url-status=live }}</ref> Originally it was thought that the Late Heavy Bombardment was a single cataclysmic impact event occurring at 3.9 Gya; this would have had the potential to sterilise all life on Earth by volatilising liquid oceans and blocking the Sun needed for photosynthesising primary producers, pushing back the earliest possible emergence of life to after the Late Heavy Bombardment.<ref>{{Cite journal |last1=Sleep |first1=Norman H. |last2=Zahnle |first2=Kevin J. |last3=Kasting |first3=James F. |last4=Morowitz |first4=Harold J. |date=December 1989 |title=Annihilation of ecosystems by large asteroid impacts on the early Earth |journal=Nature |volume=342 |issue=6246 |pages=139–142 |doi=10.1038/342139a0 |pmid=11536616 |bibcode=1989Natur.342..139S |s2cid=1137852 |url=https://www.nature.com/articles/342139a0 |archive-date=31 January 2024 |archive-url=https://web.archive.org/web/20240131154923/https://www.nature.com/articles/342139a0 |url-status=live }}</ref> However, more recent research questioned both the intensity of the Late Heavy Bombardment as well as its potential for sterilisation. Uncertainties as to whether Late Heavy Bombardment was one giant impact or a period of greater impact rates greatly changed the implication of its destructive power.<ref>{{Cite journal |last1=Fassett |first1=Caleb I. |last2=Minton |first2=David A. |date=2013-06-23 |title=Impact bombardment of the terrestrial planets and the early history of the Solar System |journal=Nature Geoscience |volume=6 |issue=7 |pages=520–524 |doi=10.1038/ngeo1841 |bibcode=2013NatGe...6..520F |url=https://www.nature.com/articles/ngeo1841 |archive-date=31 January 2024 |archive-url=https://web.archive.org/web/20240131154819/https://www.nature.com/articles/ngeo1841 |url-status=live }}</ref><ref>{{Cite journal |last1=Abramov |first1=Oleg |last2=Mojzsis |first2=Stephen J. |date=May 2009 |title=Microbial habitability of the Hadean Earth during the late heavy bombardment |journal=Nature |volume=459 |issue=7245 |pages=419–422 |doi=10.1038/nature08015 |pmid=19458721 |bibcode=2009Natur.459..419A |s2cid=3304147 |url=https://www.nature.com/articles/nature08015 |archive-date=31 January 2024 |archive-url=https://web.archive.org/web/20240131154926/https://www.nature.com/articles/nature08015 |url-status=live }}</ref> The 3.9 Ga date arose from dating of ] collected mostly near the ], biasing the age of recorded impacts.<ref>{{Cite journal |last1=Boehnke |first1=Patrick |last2=Harrison |first2=T. Mark |date=2016-09-12 |title=Illusory Late Heavy Bombardments |journal=Proceedings of the National Academy of Sciences |volume=113 |issue=39 |pages=10802–10806 |doi=10.1073/pnas.1611535113 |pmid=27621460 |pmc=5047187 |bibcode=2016PNAS..11310802B |doi-access=free }}</ref> Impact modelling of the lunar surface reveals that rather than a cataclysmic event at 3.9 Ga, multiple small-scale, short-lived periods of bombardment likely occurred.<ref>{{Cite journal |last=Zellner |first=Nicolle E. B. |date=2017-05-03 |title=Cataclysm No More: New Views on the Timing and Delivery of Lunar Impactors |journal=Origins of Life and Evolution of Biospheres |volume=47 |issue=3 |pages=261–280 |doi=10.1007/s11084-017-9536-3 |pmid=28470374 |pmc=5602003 |arxiv=1704.06694 |bibcode=2017OLEB...47..261Z }}</ref> Terrestrial data backs this idea by showing multiple periods of ejecta in the rock record both before and after the 3.9 Ga marker, suggesting that the early Earth was subject to continuous impacts that would not have had as great an impact on extinction as previously thought.<ref>{{Cite journal |last1=Lowe |first1=Donald R. |last2=Byerly |first2=Gary R. |date=2018-04-01 |title=The terrestrial record of Late Heavy Bombardment |journal=New Astronomy Reviews |volume=81 |pages=39–61 |doi=10.1016/j.newar.2018.03.002|bibcode=2018NewAR..81...39L |doi-access=free }}</ref> If the Late Heavy Bombardment was not a single cataclysmic event, the emergence of life could have taken place far before 3.9 Ga. | |||
| In a hypercycle, the ] (possibly ]) produces an ], which catalyzes the formation of another information system, in sequence until the product of the last aids in the formation of the first information system. Mathematically treated, hypercycles could create ], which through natural selection entered into a form of Darwinian evolution. A boost to hypercycle theory was the discovery that RNA, in certain circumstances forms itself into ]s, capable of catalyzing their own chemical reactions.<ref name="eigen"> thebioreview.com Retrieved on ]</ref> However, these reactions are limited to self-excisions (in which a longer RNA molecule becomes shorter), and much rarer small additions that are incapable of coding for any useful protein. The hypercycle theory is further degraded since the hypothetical RNA would require the existence of complex biochemicals such as nucleotides which are not formed under the conditions proposed by the Miller-Urey experiment. | |||
| If life evolved in the ocean at depths of more than ten meters, it would have been shielded both from late impacts and the then high levels of ultraviolet radiation from the sun. Geothermically heated oceanic crust could have yielded far more organic compounds through deep ]s than the ]s indicated.<ref>{{harvnb|Davies|1999|p=155}}</ref> The available energy is maximized at 100–150 °C, the temperatures at which ] bacteria and ] ] live.<ref>{{harvnb|Bock|Goode|1996}}</ref> | |||
| ====Wächtershäuser's hypothesis==== | |||
| {{Main|iron-sulfur world theory}} | |||
| ]]] | |||
| Another possible answer to this polymerization conundrum was provided in 1980s by ], in his ]. In this theory, he postulated the evolution of (bio)chemical pathways as fundamentals of the evolution of life. Moreover, he presented a consistent system of tracing today's biochemistry back to ancestral reactions that provide alternative pathways to the synthesis of organic building blocks from simple gaseous compounds. | |||
| ==== Earliest evidence of life ==== | |||
| In contrast to the classical Miller experiments, which depend on external sources of energy (such as simulated lightning or UV irradiation), "Wächtershäuser systems" come with a built-in source of energy, ]s of ] and other minerals (e.g. pyrite). The energy released from ] reactions of these metal sulfides is not only available for the synthesis of organic molecules, but also for the formation of ]s and ]s. It is therefore hypothesized that such systems may be able to evolve into ] of self-replicating, metabolically active entities that would predate the life forms known today. | |||
| {{main|Earliest known life forms}} | |||
| The experiment produced a relatively small yield of ] (0.4% to 12.4%) and a smaller yield of ]s (0.10%) but the authors also noted that: "under these same conditions dipeptides hydrolysed rapidly."<ref>{{cite journal |last=Huber |first=C. |coauthors=Wächterhäuser, G. |year=1998 |title=Peptides by activation of amino acids with CO on (Ni,Fe)S surfaces: implications for the origin of life |journal=Science |volume=281 |issue=5377 |pages=670–2 |doi=10.1126/science.281.5377.670 |pmid=9685253 }}</ref> | |||
| The exact timing at which life emerged on Earth is unknown. Minimum age estimates are based on evidence from the ]. The earliest physical evidence of life so far found consists of ]s in the ] of Northern Quebec, in ] rocks at least 3.77 and possibly as old as 4.32 Gya. The micro-organisms could have lived within hydrothermal vent precipitates, soon after the 4.4 Gya ] during the Hadean. The microbes resembled modern hydrothermal vent bacteria, supporting the view that abiogenesis began in such an environment.<ref name="NAT-20170301">{{cite journal |last1=Dodd |first1=Matthew S. |last2=Papineau |first2=Dominic |last3=Grenne |first3=Tor |last4=Slack |first4=John F. |last5=Rittner |first5=Martin |last6=Pirajno |first6=Franco |last7=O'Neil |first7=Jonathan |last8=Little |first8=Crispin T.S. |display-authors=3 |title=Evidence for early life in Earth's oldest hydrothermal vent precipitates |journal=] |date=1 March 2017 |volume=543 |issue=7643 |pages=60–64 |doi=10.1038/nature21377 |doi-access=free |pmid=28252057 |bibcode=2017Natur.543...60D |url=http://eprints.whiterose.ac.uk/112179/ |access-date=2 March 2017 |url-status=live |archive-url=https://web.archive.org/web/20170908201821/http://eprints.whiterose.ac.uk/112179/ |archive-date=8 September 2017}}</ref> | |||
| ====Radioactive beach hypothesis==== | |||
| Zachary Adam at the ], Seattle, claims that stronger tidal processes from a much closer moon may have concentrated grains of ] and other radioactive elements at the high water mark on primordial beaches where they may have been responsible for generating life's building blocks.<ref>{{cite news |first=Lewis |last=Dartnell |authorlink= |coauthors= |title=Life's a beach on planet Earth |curly=y |work=] |publisher= |date=2008-01-12 |accessdate= }}</ref> According to computer models reported in '']'',<ref>{{cite journal |last=Adam |first=Zachary |authorlink= |coauthors= |year=2007 |month= |title=Actinides and Life's Origins |journal=Astrobiology |volume=7 |issue=6 |pages=852–72 |doi=10.1089/ast.2006.0066 |accessdate= }}</ref> a deposit of such radioactive materials could show the same self-sustaining nuclear reaction as that found in the ] uranium ore seam in ]. Such radioactive beach sand provides sufficient energy to generate organic molecules, such as ]s and ]s from ] in water. Radioactive ] also releases soluble ] into regions between sand-grains, making it biologically "accessible". Thus amino acids, sugars and soluble phosphates can all be simultaneously produced, according to Adam. Radioactive ]s, then in greater concentrations, could have formed part of organo-metallic complexes. These complexes could have been important early ] to living processes. | |||
| Biogenic ] has been found in 3.7 Gya metasedimentary rocks from southwestern ]<ref name="NG-20131208">{{cite journal |last1=Ohtomo |first1=Yoko |last2=Kakegawa |first2=Takeshi |last3=Ishida |first3=Akizumi |last4=Nagase |first4=Toshiro |last5=Rosing |first5=Minik T. |display-authors=3 |date=January 2014 |title=Evidence for biogenic graphite in early Archaean Isua metasedimentary rocks |journal=] |volume=7 |issue=1 |pages=25–28 |bibcode=2014NatGe...7...25O |doi=10.1038/ngeo2025}}</ref> and in ] fossils from 3.49 Gya cherts in the ] region of ].<ref name="AST-20131108">{{cite journal |last1=Noffke |first1=Nora |author1-link=Nora Noffke |last2=Christian |first2=Daniel |last3=Wacey |first3=David |last4=Hazen |first4=Robert M. |author-link4=Robert Hazen |date=16 November 2013 |title=Microbially Induced Sedimentary Structures Recording an Ancient Ecosystem in the ''ca.'' 3.48 Gyo Dresser Formation, Pilbara, Western Australia |journal=] |volume=13 |issue=12 |pages=1103–1124 |bibcode=2013AsBio..13.1103N |doi=10.1089/ast.2013.1030 |pmc=3870916 |pmid=24205812}}</ref> Evidence of early life in rocks from ] Island, near the ] in southwestern Greenland, dating to 3.7 Gya, have shown biogenic ]s.<ref>{{harvnb|Davies|1999}}</ref> In other parts of the Isua supracrustal belt, graphite inclusions trapped within ] crystals are connected to the other elements of life: oxygen, nitrogen, and possibly phosphorus in the form of ], providing further evidence for life 3.7 Gya.<ref>{{cite journal |last1=Hassenkam |first1=T. |last2=Andersson |first2=M. P. |last3=Dalby |first3=K. N. |last4=Mackenzie |first4=D. M. A. |last5=Rosing |first5=M.T. |title=Elements of Eoarchean life trapped in mineral inclusions |journal=] |doi=10.1038/nature23261 |pmid=28738409 |volume=548 |issue=7665 |pages=78–81 |year=2017 |bibcode=2017Natur.548...78H |s2cid=205257931}}</ref> In the ] region of Western Australia, compelling evidence of early life was found in ]-bearing sandstone in a fossilized beach, with rounded tubular cells that oxidized sulfur by photosynthesis in the absence of oxygen.<ref>{{cite journal |last=O'Donoghue |first=James |date=21 August 2011 |title=Oldest reliable fossils show early life was a beach |journal=] |doi=10.1016/S0262-4079(11)62064-2 |volume=211 |page=13 |url=https://www.newscientist.com/article/dn20813-oldest-reliable-fossils-show-early-life-was-a-beach/ |url-status=live |archive-url=https://web.archive.org/web/20150630201918/http://www.newscientist.com/article/dn20813-oldest-reliable-fossils-show-early-life-was-a-beach.html |archive-date=30 June 2015}}</ref><ref>{{cite journal |last1=Wacey |first1=David |last2=Kilburn |first2=Matt R. |last3=Saunders |first3=Martin |last4=Cliff |first4=John |last5=Brasier |first5=Martin D. |author-link5=Martin Brasier |display-authors=3 |date=October 2011 |title=Microfossils of sulphur-metabolizing cells in 3.4-billion-year-old rocks of Western Australia |journal=] |volume=4 |issue=10 |pages=698–702 |bibcode=2011NatGe...4..698W |doi=10.1038/ngeo1238}}</ref> Carbon isotope ratios on graphite inclusions from the Jack Hills zircons suggest that life could have existed on Earth from 4.1 Gya.<ref>{{Cite journal |last1=Bell |first1=Elizabeth A. |last2=Boehnke |first2=Patrick |last3=Harrison |first3=T. Mark |last4=Mao |first4=Wendy L. |date=2015-11-24 |title=Potentially biogenic carbon preserved in a 4.1 billion-year-old zircon |journal=Proceedings of the National Academy of Sciences |volume=112 |issue=47 |pages=14518–14521 |doi=10.1073/pnas.1517557112 |pmc=4664351 |pmid=26483481 |bibcode=2015PNAS..11214518B |doi-access=free }}</ref> | |||
| John Parnell of the ] suggests that such a process could provide part of the "crucible of life" on any early wet rocky planet, so long as the planet is large enough to have generated a system of ] which brings radioactive minerals to the surface. As the early Earth is believed to have many smaller "platelets" it would provide a suitable environment for such processes.<ref>{{cite journal | |||
| | year = 2004 | |||
| | title = Mineral Radioactivity in Sands as a Mechanism for Fixation of Organic Carbon on the Early Earth | |||
| | journal = Origins of Life and Evolution of Biospheres | |||
| | last = Parnell | |||
| | first = John | |||
| | volume = 34 | |||
| | issue = 6 | |||
| | pages = 533–547 | |||
| | doi = 10.1023/B:ORIG.0000043132.23966.a1 | |||
| | url = http://www.springerlink.com/content/mp42778372jv6054/fulltext.pdf | |||
| |format=PDF}}</ref> | |||
| The Pilbara region of Western Australia contains the ] with rocks 3.48 Gya, including layered structures called ]s. Their modern counterparts are created by photosynthetic micro-organisms including ].<ref>{{cite journal |last1=Baumgartner |first1=Rafael |last2=Van Kranendonk |first2=Martin |last3= Wacey |first3=David |last4=Fiorentini |first4=Marco |last5=Saunders |first5=Martin |last6=Caruso |first6=Caruso |last7=Pages |first7=Anais |last8=Homann |first8=Martin |last9= Guagliardo |first9=Paul |display-authors=3 |year=2019 |title=Nano−porous pyrite and organic matter in 3.5-billion-year-old stromatolites record primordial life |journal= ] |volume=47 |issue=11 |pages=1039–1043 |doi=10.1130/G46365.1 |bibcode=2019Geo....47.1039B |s2cid=204258554 |url= https://discovery.ucl.ac.uk/id/eprint/10087275/1/Baumgartner%20et%20al%202019%20accepted.pdf |access-date=10 January 2021 |archive-date=5 December 2020 |archive-url=https://web.archive.org/web/20201205130046/https://discovery.ucl.ac.uk/id/eprint/10087275/1/Baumgartner%20et%20al%202019%20accepted.pdf |url-status=live }}</ref> These lie within undeformed hydrothermal-sedimentary strata; their texture indicates a biogenic origin. Parts of the Dresser formation preserve ]s on land, but other regions seem to have been shallow seas.<ref name="Djokic Van Kranendonk 2017">{{cite journal |last1=Djokic |first1=Tara |last2=Van Kranendonk |first2=Martin J. |last3=Campbell |first3=Kathleen A. |last4=Walter |first4=Malcolm R. |last5=Ward |first5=Colin R. |date=9 May 2017 |title=Earliest signs of life on land preserved in ca. 3.5 Gao hot spring deposits |journal=] |volume=8 |page=15263 |bibcode=2017NatCo...815263D |doi=10.1038/ncomms15263 |pmc=5436104 |pmid=28486437}}</ref> A molecular clock analysis suggests the LUCA emerged prior to 3.9 Gya.<ref>{{Cite journal |last1=Betts |first1=Holly C. |last2=Puttick |first2=Mark N. |last3=Clark |first3=James W. |last4=Williams |first4=Tom A. |last5=Donoghue |first5=Philip C. J. |last6=Pisani |first6=Davide |date=20 August 2018 |title=Integrated genomic and fossil evidence illuminates life's early evolution and eukaryote origin |journal=Nature Ecology & Evolution |volume=2 |issue=10 |pages=1556–1562 |doi=10.1038/s41559-018-0644-x |pmid=30127539|pmc=6152910 |bibcode=2018NatEE...2.1556B }}</ref> | |||
| ====Models to explain homochirality==== | |||
| {{Main|Homochirality}} | |||
| Some process in chemical evolution must account for the origin of ], i.e. all building blocks in living organisms having the same "handedness" (] being left-handed, nucleic acid sugars (] and ]) being right-handed, and chiral ]). Chiral molecules can be synthesized, but in the absence of a chiral source or a chiral catalyst are formed in a 50/50 mixture of both ]. This is called a ] mixture. Clark has suggested that homochirality may have started in space, as the studies of the amino acids on the ] showed L-alanine to be more than twice as frequent as its D form, and L-glutamic acid was more than 3 times prevalent than its D counterpart. It is suggested that ] has the power to destroy one ] within the ]. Noyes<ref>{{cite journal |author=Noyes HP, Bonner WA, Tomlin JA |title=On the origin of biological chirality via natural beta-decay |journal=Orig. Life |volume=8 |issue=1 |pages=21–3 |year=1977 |month=April |pmid=896189 }}</ref> showed that ] caused the breakdown of D-], in a ] mixture, and that the presence of ], present in larger amounts in organic chemicals in the early Earth environment, could have been the cause. Robert M. Hazen reports upon experiments conducted in which various chiral crystal surfaces | |||
| <gallery mode="packed" heights="160"> | |||
| ====Self-organization and replication==== | |||
| File:Stromatolites.jpg|Fossilized ]s in the Siyeh Formation, ], dated 3.5 Gya, placing them among the earliest life-forms | |||
| {{main|Self-organization}} | |||
| File:Stromatolites in Sharkbay.jpg|Modern stromatolites in ], created by photosynthetic ] | |||
| </gallery> | |||
| == Producing molecules: prebiotic synthesis == | |||
| While features of self-organization and self-replication are often considered the hallmark of living systems, there are many instances of abiotic molecules exhibiting such characteristics under proper conditions. For example Martin and Russel show that physical compartmentation by ] from the environment and self-organization of self-contained ] reactions are the most conserved attributes of living things, and they argue therefore that inorganic matter with such attributes would be life's most likely last common ancestor.<ref>{{cite journal |last=Martin |first=William |coauthors=Russel, Michael J. |year=2003 |title=On the origins of cells: a hypothesis for the evolutionary transitions from abiotic geochemistry to chemoautotrophic prokaryotes, and from prokaryotes to nucleated cells |journal=Phil. Trans. R. Soc. B |volume=358 |issue=1429 |pages=59–85 |doi=10.1098/rstb.2002.1183 }}</ref> | |||
| {{further|Nucleosynthesis}} | |||
| Virus self-assembly within host cells has implications for the study of the origin of life,<ref name=pmid16984643>{{cite journal | |||
| | author = Koonin EV, Senkevich TG, Dolja VV | |||
| | title = The ancient Virus World and evolution of cells | |||
| | journal = Biol. Direct | |||
| | volume = 1 | |||
| | page = 29 | |||
| | year = 2006 | |||
| | pmid = 16984643 | |||
| | pmc = 1594570 | |||
| | doi = 10.1186/1745-6150-1-29 | |||
| | url = http://www.biology-direct.com/content/1//29 | |||
| | accessdate = 2008-10-20 | |||
| | pages = 29 | |||
| }}</ref> as it lends further credence to the hypothesis that life could have started as self-assembling organic molecules.<ref name="pmid16044244">{{cite journal | |||
| | author = Vlassov AV, Kazakov SA, Johnston BH, Landweber LF | |||
| | title = The RNA world on ice: a new scenario for the emergence of RNA information | |||
| | journal = J. Mol. Evol. | |||
| | volume = 61 | |||
| | issue = 2 | |||
| | pages = 264–73 | |||
| | year = 2005 | |||
| | month = August | |||
| | pmid = 16044244 | |||
| | doi = 10.1007/s00239-004-0362-7 | |||
| | accessdate = 2008-10-20 | |||
| }}</ref> | |||
| All ]s derive from stellar nucleosynthesis except for hydrogen and some helium and lithium. Basic chemical ingredients of life – the ] (CH), the carbon-hydrogen positive ion (CH+) and the carbon ion (C+) – can be produced by ] from stars.<ref name="NASA-20161012">{{cite web |last=Landau |first=Elizabeth |title=Building Blocks of Life's Building Blocks Come From Starlight |url=http://www.jpl.nasa.gov/news/news.php?feature=6645 |date=12 October 2016 |work=] |access-date=13 October 2016 |url-status=live |archive-url=https://web.archive.org/web/20161013135018/http://www.jpl.nasa.gov/news/news.php?feature=6645 |archive-date=13 October 2016}}</ref> Complex molecules, including organic molecules, form naturally both in space and on planets.<ref name="Ehrenfreund2010"/> Organic molecules on the early Earth could have had either terrestrial origins, with organic molecule synthesis driven by impact shocks or by other energy sources, such as ultraviolet light, ] coupling, or electrical discharges; or extraterrestrial origins (]), with organic molecules formed in ] raining down on to the planet.<ref>{{cite journal |last1=Geballe |first1=Thomas R. |last2=Najarro |first2=Francisco |last3=Figer |first3=Donald F. |author-link3=Donald Figer |last4=Schlegelmilch |first4=Barret W. |last5=de la Fuente |first5=Diego |display-authors=3 |date=10 November 2011 |title=Infrared diffuse interstellar bands in the Galactic Centre region |journal=] |volume=479 |issue=7372 |pages=200–202 |arxiv=1111.0613 |bibcode=2011Natur.479..200G |doi=10.1038/nature10527 |pmid=22048316 |s2cid=17223339 |ref=none}}</ref><ref>{{harvnb|Klyce|2001}}</ref> | |||
| ===From organic molecules to protocells=== | |||
| The question "How do simple organic molecules form a protocell?" is largely unanswered but there are many hypotheses. Some of these postulate the early appearance of nucleic acids ("]s-first") whereas others postulate the evolution of biochemical reactions and pathways first ("]-first"). Recently, trends are emerging to create hybrid models that combine aspects of both. | |||
| === Observed extraterrestrial organic molecules === | |||
| ===="Genes first" models: the RNA world==== | |||
| {{main|RNA world hypothesis}} | |||
| {{see also|List of interstellar and circumstellar molecules|Pseudo-panspermia}} | |||
| The ] describes an early Earth with self-replicating and catalytic RNA but no DNA or proteins. This has spurred scientists to try to determine if relatively short ] molecules could have spontaneously formed that were capable of catalyzing their own continuing replication.<ref>{{cite journal |author=Ma W, Yu C, Zhang W, Hu J |title=Nucleotide synthetase ribozymes may have emerged first in the RNA world |journal=RNA (New York, N.Y.) |volume=13 |issue=11 |pages=2012–9 |year=2007 |month=November |pmid=17878321 |pmc=2040096 |doi=10.1261/rna.658507}}</ref> A number of hypotheses of modes of formation have been put forward. Early cell membranes could have formed spontaneously from ]s, protein-like molecules that are produced when amino acid solutions are heated–when present at the correct concentration in aqueous solution, these form microspheres which are observed to behave similarly to membrane-enclosed compartments. Other possibilities include systems of chemical reactions taking place within ] substrates or on the surface of ] rocks. Factors supportive of an important role for RNA in early life include its ability to act both to store information and catalyse chemical reactions (as a ]); its many important roles as an intermediate in the expression and maintenance of the genetic information (in the form of ]) in modern organisms; and the ease of chemical synthesis of at least the components of the molecule under conditions approximating the early Earth. Relatively short RNA molecules which can duplicate others have been artificially produced in the lab.<ref>{{cite journal |last=Johnston |first=W. K. |authorlink= |coauthors=''et al.'' |year=2001 |month= |title=RNA-Catalyzed RNA Polymerization: Accurate and General RNA-Templated Primer Extension |journal=Science |volume=292 |issue=5520 |pages=1319–1325 |doi=10.1126/science.1060786 |pmid=11358999 }}</ref> Such replicase RNA, which functions as both code and catalyst provides a template upon which copying can occur. ] has shown that certain catalytic RNAs can, indeed, join smaller RNA sequences together, creating the potential, in the right conditions for self-replication. If these were present, ] would favour the proliferation of such self-catalysing structures, to which further functionalities could be added.<ref>{{cite web|first=Jack W. |last=Szostak |url=http://www.hhmi.org/research/investigators/szostak.html |title=The Origins of Function in Biological Nucleic Acids, Proteins, and Membranes |publisher=HHMI |date=June 4, 2008 |accessdate=2008-11-29}}</ref> Lincoln and Joyce identified an RNA enzyme capable of self sustained replication.<ref>{{cite journal | |||
| | last = Lincoln | |||
| | first = Tracey A. | |||
| | coauthors = Joyce, Gerald F. | |||
| | date = January 8, 2009 | |||
| | title = Self-Sustained Replication of an RNA Enzyme | |||
| | journal = Science | |||
| | volume = | |||
| | issue = | |||
| | pages = | |||
| | publisher = American Association for the Advancement of Science | |||
| | location = New York | |||
| | issn = 1095-9203 | |||
| | pmid = 19131595 | |||
| | doi = 10.1126/science.1167856 | |||
| | url = http://www.sciencemag.org/cgi/content/abstract/1167856 | |||
| | accessdate = 2009-01-13}} | |||
| </ref> | |||
| An organic compound is a chemical whose molecules contain carbon. Carbon is abundant in the Sun, stars, comets, and in the ]s of most planets of the Solar System.<ref name="NASA-20140221">{{cite web |url=http://www.nasa.gov/ames/need-to-track-organic-nano-particles-across-the-universe-nasas-got-an-app-for-that/ |title=Need to Track Organic Nano-Particles Across the Universe? NASA's Got an App for That |last=Hoover |first=Rachel |date=21 February 2014 |website=] |publisher=] |access-date=22 June 2015 |url-status=live |archive-url=https://web.archive.org/web/20150906061428/http://www.nasa.gov/ames/need-to-track-organic-nano-particles-across-the-universe-nasas-got-an-app-for-that/ |archive-date=6 September 2015}}</ref> Organic compounds are relatively common in space, formed by "factories of complex molecular synthesis" which occur in molecular clouds and ]s, and chemically evolve after reactions are initiated mostly by ].<ref name="Ehrenfreund2010">{{cite journal |last1=Ehrenfreund |first1=Pascale |last2=Cami |first2=Jan |date=December 2010 |title=Cosmic carbon chemistry: from the interstellar medium to the early Earth. |journal=] |volume=2 |issue=12 |page=a002097 |doi=10.1101/cshperspect.a002097 |pmc=2982172 |pmid=20554702}}</ref><ref>{{cite journal |last1=Goncharuk |first1=Vladislav V. |last2=Zui |first2=O. V. |date=February 2015 |title=Water and carbon dioxide as the main precursors of organic matter on Earth and in space |journal=Journal of Water Chemistry and Technology |volume=37 |issue=1 |pages=2–3 |doi=10.3103/S1063455X15010026 |bibcode=2015JWCT...37....2G |s2cid=97965067}}</ref><ref>{{cite journal |last1=Abou Mrad |first1=Ninette |last2=Vinogradoff |first2=Vassilissa |last3=Duvernay |first3=Fabrice |last4=Danger |first4=Grégoire |last5=Theulé |first5=Patrice |last6=Borget |first6=Fabien |last7=Chiavassa |first7=Thierry |display-authors=3 |year=2015 |title=Laboratory experimental simulations: Chemical evolution of the organic matter from interstellar and cometary ice analogs |url=http://popups.ulg.ac.be/0037-9565/index.php?id=4621&file=1 |journal=Bulletin de la Société Royale des Sciences de Liège |volume=84 |pages=21–32 |bibcode=2015BSRSL..84...21A |access-date=6 April 2015 |url-status=live |archive-url=https://web.archive.org/web/20150413050621/http://popups.ulg.ac.be/0037-9565/index.php?id=4621&file=1 |archive-date=13 April 2015}}</ref> ] and ] nucleobases including ], ], ], ], and ] have been found in ]s. These could have provided the materials for DNA and ] to form on the ].<ref name="NC-20220426">{{cite journal |last=Oba |first=Yasuhiro |display-authors=|title=Identifying the wide diversity of extraterrestrial purine and pyrimidine nucleobases in carbonaceous meteorites |date=26 April 2022 |journal=] |volume=13 |number=2008 |page=2008 |doi=10.1038/s41467-022-29612-x |pmid=35473908 |pmc=9042847 |bibcode=2022NatCo..13.2008O |s2cid=248402205}}</ref> The amino acid ] was found in material ejected from comet ]; it had earlier been detected in meteorites.<ref>{{cite news |last=<!--Staff writer(s); no by-line.--> |date=18 August 2009 |title='Life chemical' detected in comet |url=http://news.bbc.co.uk/2/hi/science/nature/8208307.stm |work=BBC News |location=London |access-date=23 June 2015 |url-status=live |archive-url=https://web.archive.org/web/20150525071228/http://news.bbc.co.uk/2/hi/science/nature/8208307.stm |archive-date=25 May 2015}}</ref> Comets are encrusted with dark material, thought to be a ]-like organic substance formed from simple carbon compounds under ionizing radiation. A rain of material from comets could have brought such complex organic molecules to Earth.<ref>{{cite journal |last1=Thompson |first1=William Reid |last2=Murray |first2=B. G. |last3=Khare |first3=Bishun Narain |author-link3=Bishun Khare |last4=Sagan |first4=Carl |author4-link=Carl Sagan |date=30 December 1987 |title=Coloration and darkening of methane clathrate and other ices by charged particle irradiation: Applications to the outer solar system |journal=] |volume=92 |issue=A13 |pages=14933–14947 |bibcode=1987JGR....9214933T |doi=10.1029/JA092iA13p14933 |pmid=11542127}}</ref><ref>{{cite journal |last1=Goldman |first1=Nir |last2=Tamblyn |first2=Isaac |date=20 June 2013 |title=Prebiotic Chemistry within a Simple Impacting Icy Mixture |journal=] |volume=117 |issue=24 |pages=5124–5131 |doi=10.1021/jp402976n |pmid=23639050 |bibcode=2013JPCA..117.5124G |s2cid=5144843 |url=http://nparc.nrc-cnrc.gc.ca/eng/view/fulltext/?id=e89d2ac7-4cf8-40e0-bcc9-3c53f68ed70a |access-date=29 August 2019 |archive-date=21 July 2018 |archive-url=https://web.archive.org/web/20180721183453/http://nparc.nrc-cnrc.gc.ca/eng/view/fulltext/?id=e89d2ac7-4cf8-40e0-bcc9-3c53f68ed70a |url-status=live }}</ref><ref name="Follmann2009"/> It is estimated that during the Late Heavy Bombardment, meteorites may have delivered up to five million ]s of organic prebiotic elements to Earth per year.<ref name="Follmann2009"/> Currently 40,000 tons of cosmic dust falls to Earth each year.<ref>https://www.astronomy.com/science/how-much-dust-falls-on-earth-each-year-does-it-affect-our-planets-gravity/</ref> | |||
| Researchers have pointed out difficulties for the abiotic synthesis of nucleotides from ] and ].<ref>{{cite journal |last=Orgel |first=L. |authorlink= |coauthors= |year=1994 |month= |title=The origin of life on earth |journal=Scientific American |volume=271 |issue=4 |page=81 |doi= }}</ref> Cytosine has a ] of 19 days at {{convert|100|°C|°F}} and 17,000 years in freezing water.<ref>{{cite journal |last=Levy |first=Matthew |authorlink= |coauthors=Miller, Stanley L. |year=1998 |month= |title=The stability of the RNA bases: Implications for the origin of life |journal=PNAS |volume=95 |issue= |pages=7933–7938 |id= |doi=10.1073/pnas.95.14.7933|pmid=9653118 }}</ref> Larralde et al., say that "the generally accepted prebiotic synthesis of ribose, the formose reaction, yields numerous sugars without any selectivity."<ref>{{cite journal |last=Larralde |first=R. |authorlink= |coauthors=Robertson, M. P.; Miller, S. L. |year=1995 |month= |title=Rates of Decomposition of Ribose and Other Sugars: Implications for Chemical Evolution |journal=PNAS |volume=92 |issue=18 |pages=8158–8160 |id= |doi=10.1073/pnas.92.18.8158|pmid=7667262 }}</ref> and they conclude that their "results suggest that the backbone of the first genetic material could not have contained ribose or other sugars because of their instability." The ester linkage of ribose and phosphoric acid in RNA is known to be prone to hydrolysis.<ref>{{cite journal |last=Lindahl |first=Tomas |authorlink= |coauthors= |year=1993 |month= |title=Instability and decay of the primary structure of DNA |journal=Nature |volume=362 |issue=6422 |pages=709–715 |doi=10.1038/362709a0 }}</ref> | |||
| ==== Polycyclic aromatic hydrocarbons==== | |||
| A slightly different version of the RNA-world hypothesis is that a different type of ], such as ], ] or ], was the first one to emerge as a self-reproducing molecule, to be replaced by RNA only later.<ref>{{cite journal |last=Orgel |first=Leslie |authorlink= |coauthors= |year=2000 |month= |title=A Simpler Nucleic Acid |journal=Science |volume=290 |issue=5495 |pages=1306–1307 |doi=10.1126/science.290.5495.1306 |pmid=11185405 }}</ref><ref>{{cite journal |last=Nelson |first=K. E. |authorlink= |coauthors=Levy, M.; Miller, S. L. |year=2000 |month= |title=Peptide nucleic acids rather than RNA may have been the first genetic molecule |journal=PNAS |volume=97 |issue=8 |pages=3868–3871 |doi= 10.1073/pnas.97.8.3868|url=http://www.pnas.org/cgi/content/abstract/97/8/3868 |accessdate= |format=abstract|pmid=10760258 }}</ref> Pyrimidine ribonucleosides and their respective nucleotides have been prebiotically synthesised by a sequence of reactions which by-pass the free sugars, and are assembled in a stepwise fashion by going against the dogma that nitrogenous and oxygenous chemistries should be avoided. In a series of publications, The Sutherland Group at the School of Chemistry, University of Manchester have demonstrated high yielding routes to cytidine and uridine ribonucleotides built from small 2 and 3 carbon fragments such as glycolaldehyde, glyceraldehyde or glyceraldehyde-3-phosphate, cyanamide and cyanoacetylene. One of the steps in this sequence allows the isolation of enantiopure ribose aminooxazoline if the enantiomeric excess of glyceraldehyde is 60 % or greater.<ref>{{cite journal |author=Anastasi C, Crowe MA, Powner MW, Sutherland JD |title=Direct Assembly of Nucleoside Precursors from Two- and Three-Carbon Units |journal=Angewandte Chemie International Edition |volume=45 |issue=37 |pages=6176–9 |year=2006 |doi=10.1002/anie.200601267 |url=http://www3.interscience.wiley.com/journal/112752038/abstract}}</ref> This can be viewed as a prebiotic purification step, where the said compound spontaneously crystallised out from a mixture of the other pentose aminooxazolines. Ribose aminooxazoline can then react with cyanoacetylene in a mild and highly efficient manner to give the alpha cytidine ribonucleotide. Photoanomerization with UV light allows for inversion about the 1' anomeric centre to give the correct beta stereochemistry.<ref>{{cite journal |author=Powner MW, Sutherland JD |title=Potentially Prebiotic Synthesis of Pyrimidine β-D-Ribonucleotides by Photoanomerization/Hydrolysis of α-D-Cytidine-2-Phosphate |journal=ChemBioChem |volume=9 |issue=15 |pages=2386–7 |year=2008 |doi=10.1002/cbic.200800391 |url=http://www3.interscience.wiley.com/journal/121410594/abstract}}</ref> In 2009 they showed that the same simple building blocks allow access, via phosphate controlled nucleobase elaboration, to 2',3'-cyclic pyrimidine nucleotides directly, which are known to be able to polymerise into RNA. This paper also highlights the possibility for the photo-sanitization of the pyrimidine-2',3'-cyclic phosphates. <ref>{{cite journal |author=Powner MW, Gerland B, Sutherland JD |title=Synthesis of activated pyrimidine ribonucleotides in prebiotically plausible conditions |journal=Nature |volume=459 |issue=7244 |pages=239–42 |year=2009 |month=May |pmid=19444213 |doi=10.1038/nature08013 }}</ref> James Ferris's studies have shown that clay minerals of ] will catalyze the formation of RNA in aqueous solution, by joining activated mono RNA nucleotides to join together to form longer chains.<ref>{{cite journal |author=Huang W, Ferris JP |title=One-step, regioselective synthesis of up to 50-mers of RNA oligomers by montmorillonite catalysis |journal=J. Am. Chem. Soc. |volume=128 |issue=27 |pages=8914–9 |year=2006 |month=July |pmid=16819887 |doi=10.1021/ja061782k }}</ref> Although these chains have random sequences, the possibility that one sequence began to non-randomly increase its frequency by increasing the speed of its catalysis is possible to "kick start" biochemical evolution. | |||
| ] is inside the ], in the ] ].<br/>Green areas show regions where radiation from hot stars collided with large molecules and small dust grains called "]s" (PAHs), causing them to ]. ], 2018.]] | |||
| ===="Metabolism first" models: iron-sulfur world and others==== | |||
| Several models reject the idea of the self-replication of a "naked-gene" and postulate the emergence of a primitive metabolism which could provide an environment for the later emergence of RNA replication. | |||
| ]s (PAH) are the most common and abundant polyatomic molecules in the ], and are a major store of carbon.<ref name="NASA-20140221"/><ref>{{cite web |url=http://www.astrochem.org/pahdb/ |title=NASA Ames PAH IR Spectroscopic Database |publisher=NASA |access-date=17 June 2015 |url-status=live |archive-url=https://web.archive.org/web/20150629185734/http://www.astrochem.org/pahdb/ |archive-date=29 June 2015}}</ref><ref name="AJ-20051010">{{cite journal |last1=Hudgins |first1=Douglas M. |last2=Bauschlicher |first2=Charles W. Jr. |last3=Allamandola |first3=Louis J. |date=10 October 2005 |title=Variations in the Peak Position of the 6.2 μm Interstellar Emission Feature: A Tracer of N in the Interstellar Polycyclic Aromatic Hydrocarbon Population |journal=] |volume=632 |pages=316–332 |issue=1 |bibcode=2005ApJ...632..316H |doi=10.1086/432495 |citeseerx=10.1.1.218.8786 |s2cid=7808613}}</ref><ref name="NASA-20110413">{{cite web |last1=Des Marais |first1=David J. |last2=Allamandola |first2=Louis J. |last3=Sandford |first3=Scott |author-link3=Scott Sandford |last4=Mattioda |first4=Andrew |last5=Gudipati |first5=Murthy |last6=Roser |first6=Joseph |last7=Bramall |first7=Nathan |last8=Nuevo |first8=Michel |last9=Boersma |first9=Christiaan |last10=Bernstein |first10=Max |last11=Peeters |first11=Els |first14=Jason |last14=Dworkin |first13=Jamie Elsila |last13=Cook |first12=Jan |last12=Cami |display-authors=3 |year=2009 |title=Cosmic Distribution of Chemical Complexity |website=Ames Research Center |publisher=NASA |location=Mountain View, California |url=http://amesteam.arc.nasa.gov/Research/cosmic.html |access-date=24 June 2015 |archive-url=https://web.archive.org/web/20140227184503/http://amesteam.arc.nasa.gov/Research/cosmic.html |archive-date=27 February 2014}}</ref> They seem to have formed shortly after the Big Bang,<ref name="SP-20051018"/><ref name="AJ-20051010"/><ref name="NASA-20110413"/> and are associated with ] and ]s.<ref name="NASA-20140221"/> They are a likely constituent of Earth's primordial sea.<ref name="SP-20051018">{{cite news |last=Carey |first=Bjorn |date=18 October 2005 |title=Life's Building Blocks 'Abundant in Space' |url=http://www.space.com/1686-life-building-blocks-abundant-space.html |website=Space.com |location=Watsonville, California |publisher=] |access-date=23 June 2015 |url-status=live |archive-url=https://web.archive.org/web/20150626223942/http://www.space.com/1686-life-building-blocks-abundant-space.html |archive-date=26 June 2015}}</ref><ref name="AJ-20051010"/><ref name="NASA-20110413"/> PAHs have been detected in ]e,<ref name="AJL-20101120">{{cite journal |last1=García-Hernández |first1=Domingo. A. |last2=Manchado |first2=Arturo |last3=García-Lario |first3=Pedro |last4=Stanghellini |first4=Letizia |last5=Villaver |first5=Eva |last6=Shaw |first6=Richard A. |last7=Szczerba |first7=Ryszard |last8=Perea-Calderón |first8=Jose Vicente |display-authors=3 |date=20 November 2010 |title=Formation of Fullerenes in H-Containing Planetary Nebulae |journal=] |volume=724 |issue=1 |pages=L39–L43 |arxiv=1009.4357 |bibcode=2010ApJ...724L..39G |doi=10.1088/2041-8205/724/1/L39 |s2cid=119121764}}</ref> and in the ], in comets, and in meteorites.<ref name="NASA-20140221"/> | |||
| One of the earliest incarnations of this idea was put forward in 1924 with ]'s notion of primitive self-replicating ] which predated the discovery of the structure of DNA. More recent variants in the 1980s and 1990s include ]'s ] and models introduced by ] based on the chemistry of ]s. More abstract and theoretical arguments for the plausibility of the emergence of metabolism without the presence of genes include a mathematical model introduced by ] in the early 1980s and ]'s notion of collectively ]s, discussed later in that decade. | |||
| A star, HH 46-IR, resembling the sun early in its life, is surrounded by a disk of material which contains molecules including cyanide compounds, ]s, and carbon monoxide. PAHs in the interstellar medium can be transformed through ], ], and ] to more complex organic compounds used in living cells.<ref name="AJL-20120901">{{cite journal |last1=Gudipati |first1=Murthy S. |last2=Yang |first2=Rui |date=1 September 2012 |title=In-situ Probing of Radiation-induced Processing of Organics in Astrophysical Ice Analogs – Novel Laser Desorption Laser Ionization Time-of-flight Mass Spectroscopic Studies |journal=] |volume=756 |issue=1 |bibcode=2012ApJ...756L..24G |doi=10.1088/2041-8205/756/1/L24 |page=L24 |s2cid=5541727}}</ref> | |||
| However, the idea that a closed metabolic cycle, such as the reductive ], could form spontaneously (proposed by Günter Wächtershäuser) remains debated. In an article entitled "Self-Organizing Biochemical Cycles",<ref>{{cite journal |author=Orgel LE |title=Self-organizing biochemical cycles |journal=Proc. Natl. Acad. Sci. U.S.A. |volume=97 |issue=23 |pages=12503–7 |year=2000 |month=November |pmid=11058157 |pmc=18793 |doi=10.1073/pnas.220406697 |url=http://www.pnas.org/cgi/pmidlookup?view=long&pmid=11058157}}</ref> the late ] summarized his analysis of the proposal by stating, "There is at present no reason to expect that multistep cycles such as the reductive citric acid cycle will self-organize on the surface of FeS/FeS2 or some other mineral." It is possible that another type of metabolic pathway was used at the beginning of life. For example, instead of the reductive citric acid cycle, the "open" ] pathway (another one of the five recognised ways of carbon dioxide fixation in nature today) would be compatible with the idea of self-organisation on a metal sulfide surface. The key enzyme of this pathway, carbon monoxide dehydrogenase/acetyl-CoA synthase harbours mixed nickel-iron-sulfur clusters in its reaction centers and catalyses the formation of acetyl-CoA (which may be regarded as a modern form of acetyl-thiol) in a single step. | |||
| ==== |
==== Nucleobases and nucleotides ==== | ||
| Waves breaking on the shore create a delicate foam composed of bubbles. Winds sweeping across the ocean have a tendency to drive things to shore, much like driftwood collecting on the beach. It is possible that organic molecules were concentrated on the shorelines in much the same way. Shallow coastal waters also tend to be warmer, further concentrating the molecules through ]. While bubbles composed mostly of water burst quickly, water containing ] forms much more stable bubbles, lending more time to the particular bubble to perform these crucial reactions. | |||
| {{further|Nucleobase|Nucleotide}} | |||
| Amphiphiles are oily compounds containing a ] head on one or both ends of a ] molecule. Some amphiphiles have the tendency to spontaneously form membranes in water. A spherically closed membrane contains water and is a hypothetical precursor to the modern cell membrane. If a ] would increase the integrity of its parent bubble, that bubble had an advantage, and was placed at the top of the ] waiting list. Primitive reproduction can be envisioned when the bubbles burst, releasing the results of the 'experiment' into the surrounding medium. Once enough of the 'right stuff' was released into the medium, the development of the first ], ], and multicellular organisms could be achieved.<ref>{{cite book |author=Panno, Joseph |title=The cell: evolution of the first organism |publisher=Facts on File |location=New York |year=2005 |isbn=0-8160-4946-7 }}</ref> | |||
| Organic compounds introduced on Earth by ] can help to form complex molecules, thanks to their peculiar ] activities.<ref name="Lincei">{{cite journal |last=Gallori |first=Enzo |title=Astrochemistry and the origin of genetic material |journal=Rendiconti Lincei |date=June 2011 |volume=22 |issue=2 |pages=113–118 |doi=10.1007/s12210-011-0118-4 |s2cid=96659714}} "Paper presented at the Symposium 'Astrochemistry: molecules in space and time' (Rome, 4–5 November 2010), sponsored by Fondazione 'Guido Donegani', Accademia Nazionale dei Lincei."</ref><ref>{{cite journal |last=Martins |first=Zita |author-link=Zita Martins |date=February 2011 |title=Organic Chemistry of Carbonaceous Meteorites |journal=] |volume=7 |issue=1 |pages=35–40 |doi=10.2113/gselements.7.1.35|bibcode=2011Eleme...7...35M }}</ref> The RNA component uracil and related molecules, including ], in the ] were likely formed extraterrestrially, as suggested by studies of <sup>12</sup>C/<sup>13</sup>C ].<ref name="Murch_base">{{cite journal |last1=Martins |first1=Zita |last2=Botta |first2=Oliver |last3=Fogel |first3=Marilyn L. |last4=Sephton |first4=Mark A. |last5=Glavin |first5=Daniel P. |last6=Watson |first6=Jonathan S. |last7=Dworkin |first7=Jason P. |last8=Schwartz |first8=Alan W. |last9=Ehrenfreund |first9=Pascale |display-authors=3 |date=15 June 2008 |title=Extraterrestrial nucleobases in the Murchison meteorite |journal=] |volume=270 |issue=1–2 |pages=130–136 |bibcode=2008E&PSL.270..130M |arxiv=0806.2286 |doi=10.1016/j.epsl.2008.03.026 |s2cid=14309508}}</ref> NASA studies of meteorites suggest that all four DNA nucleobases (adenine, guanine and related organic molecules) have been formed in outer space.<ref name="Lincei"/><ref name="Callahan">{{cite journal |last1=Callahan |first1=Michael P. |last2=Smith |first2=Karen E. |last3=Cleaves |first3=H. James II |last4=Ruzica |first4=Josef |last5=Stern |first5=Jennifer C. |last6=Glavin |first6=Daniel P. |last7=House |first7=Christopher H. |last8=Dworkin |first8=Jason P. |display-authors=3 |date=23 August 2011 |title=Carbonaceous meteorites contain a wide range of extraterrestrial nucleobases |journal=] |volume=108 |issue=34 |pages=13995–13998 |bibcode=2011PNAS..10813995C |doi=10.1073/pnas.1106493108 |pmc=3161613 |pmid=21836052 |doi-access=free}}</ref><ref name="Steigerwald">{{cite web |url=http://www.nasa.gov/topics/solarsystem/features/dna-meteorites.html |title=NASA Researchers: DNA Building Blocks Can Be Made in Space |last=Steigerwald |first=John |date=8 August 2011 |work=] |publisher=NASA |access-date=23 June 2015 |url-status=live |archive-url=https://web.archive.org/web/20150623004556/http://www.nasa.gov/topics/solarsystem/features/dna-meteorites.html |archive-date=23 June 2015}}</ref> The ] permeating the universe contains complex organics ("amorphous organic solids with a mixed ]–] structure") that could be created rapidly by stars.<ref name="Nature-20111026">{{cite journal |last1=Kwok |first1=Sun |author-link=Sun Kwok |last2=Zhang |first2=Yong |date=3 November 2011 |title=Mixed aromatic–aliphatic organic nanoparticles as carriers of unidentified infrared emission features |journal=] |volume=479 |issue=7371 |pages=80–83 |bibcode=2011Natur.479...80K |doi=10.1038/nature10542 |pmid=22031328 |s2cid=4419859}}</ref> ], a sugar molecule and RNA precursor, has been detected in regions of space including around ]s and on meteorites.<ref>{{cite journal |last1=Jørgensen |first1=Jes K. |last2=Favre |first2=Cécile |last3=Bisschop |first3=Suzanne E. |last4=Bourke |first4=Tyler L. |last5=van Dishoeck |first5=Ewine F. |author-link5=Ewine van Dishoeck |last6=Schmalzl |first6=Markus |display-authors=3 |date=2012 |title=Detection of the simplest sugar, glycolaldehyde, in a solar-type protostar with ALMA |url=http://www.eso.org/public/archives/releases/sciencepapers/eso1234/eso1234a.pdf |journal=] |volume=757 |issue=1 |arxiv=1208.5498 |bibcode=2012ApJ...757L...4J |doi=10.1088/2041-8205/757/1/L4 |access-date=2015-06-23 |page=L4 |s2cid=14205612 |url-status=live |archive-url=https://web.archive.org/web/20150924021240/http://www.eso.org/public/archives/releases/sciencepapers/eso1234/eso1234a.pdf |archive-date=24 September 2015}}</ref><ref name="PNAS-20191113">{{cite journal |last1=Furukawa |first1=Yoshihiro |last2=Chikaraishi |first2=Yoshito |last3=Ohkouchi |first3=Naohiko |last4=Ogawa |first4=Nanako O. |last5=Glavin |first5=Daniel P. |last6=Dworkin |first6=Jason P. |last7=Abe |first7=Chiaki |last8=Nakamura |first8=Tomoki |display-authors=3 |date=2019-11-13 |title=Extraterrestrial ribose and other sugars in primitive meteorites |journal=] |volume=116 |issue=49 |pages=24440–24445 |doi=10.1073/pnas.1907169116 |pmid=31740594 |pmc=6900709 |bibcode=2019PNAS..11624440F |doi-access=free}}</ref> | |||
| Similarly, bubbles formed entirely out of protein-like molecules, called ]s, will form spontaneously under the right conditions. But they are not a likely precursor to the modern cell membrane, as cell membranes are composed primarily of lipid compounds rather than amino-acid compounds (for types of membrane spheres associated with abiogenesis, see ], ], ]). | |||
| === Laboratory synthesis === | |||
| A recent model by Fernando and Rowe<ref></ref> suggests that the enclosure of an autocatalytic non-enzymatic metabolism within protocells may have been one way of avoiding the side-reaction problem that is typical of metabolism first models. | |||
| As early as the 1860s, experiments demonstrated that biologically relevant molecules can be produced from interaction of simple carbon sources with abundant inorganic catalysts. The spontaneous formation of complex polymers from abiotically generated monomers under the conditions posited by the "soup" theory is not straightforward. Besides the necessary basic organic monomers, compounds that would have prohibited the formation of polymers were also formed in high concentration during the Miller–Urey and ] experiments.<ref>{{cite journal |last1=Oró |first1=Joan |last2=Kimball |first2=Aubrey P. |date=February 1962 |title=Synthesis of purines under possible primitive earth conditions: II. Purine intermediates from hydrogen cyanide |journal=] |volume=96 |issue=2 |pages=293–313 |doi=10.1016/0003-9861(62)90412-5 |pmid=14482339}}</ref> Biology uses essentially 20 amino acids for its coded protein enzymes, representing a very small subset of the structurally possible products. Since life tends to use whatever is available, an explanation is needed for why the set used is so small.<ref>{{cite journal |last1=Cleaves II |first1=Henderson |year=2010 |title=The origin of the biologically coded amino acids |journal=] |volume=263 |issue=4 |pages=490–498 |doi=10.1016/j.jtbi.2009.12.014 |pmid=20034500 |bibcode=2010JThBi.263..490C}}</ref> Formamide is attractive as a medium that potentially provided a source of amino acid derivatives from simple aldehyde and nitrile feedstocks.<ref>{{cite journal|author=Green, N. J., Russell, D. A., Tanner, S. H., Sutherland, J. D.|title=Prebiotic Synthesis of N-Formylaminonitriles and Derivatives in Formamide|journal=Journal of the American Chemical Society|year=2023|volume=145|issue=19 |pages=10533–10541|doi=10.1021/jacs.2c13306|pmid=37146260|doi-access=free|pmc=10197134|bibcode=2023JAChS.14510533G }}</ref> | |||
| ==Other models== | |||
| ===Autocatalysis=== | |||
| In 1995 ] proposed that life initially arose as autocatalytic chemical networks.<ref>{{cite book|author=Stuart Kauffman|title=At Home in the Universe: The Search for the Laws of Self-Organization and Complexity|isbn=0195095995|publisher=Oxford University Press|date=1995}}</ref> | |||
| ==== Sugars ==== | |||
| ] ] ] wrote about ] as a potential explanation for the origin of life in his 2004 book '']''. Autocatalysts are substances which catalyze the production of themselves, and therefore have the property of being a simple molecular replicator. In his book, Dawkins cites experiments performed by ] and his colleagues at the ] in ] in which they combined ] and ] with the autocatalyst ] (AATE). One system from the experiment contained variants of AATE which catalysed the synthesis of themselves. This experiment demonstrated the possibility that autocatalysts could exhibit competition within a population of entities with heredity, which could be interpreted as a rudimentary form of ]. | |||
| ] | |||
| ===Clay theory=== | |||
| A model for the origin of life based on ] was forwarded by A. ] of the ] in 1985 and explored as a plausible illustration by several other scientists, including ]<ref>{{cite book | first = Richard | last = Dawkins | authorlink = Richard Dawkins | title = The Blind Watchmaker | publisher = W. W. Norton & Company, Inc | location = New York | origyear = 1986 | year = 1996 | isbn = 0-393-31570-3 | pages = 148-161 }}</ref>. ] postulates that complex organic molecules arose gradually on a pre-existing, non-organic replication platform—silicate crystals in solution. Complexity in companion molecules developed as a function of selection pressures on types of clay crystal is then ] to serve the replication of organic molecules independently of their silicate "launch stage". | |||
| ] showed in 1861 that the ] created sugars including tetroses, pentoses, and hexoses when ] is heated under basic conditions with divalent metal ions like calcium. R. Breslow proposed that the reaction was autocatalytic in 1959.<ref>{{cite journal |last=Breslow |first=R. |year=1959 |title=On the Mechanism of the Formose Reaction |journal=] |volume=1 |issue=21 |pages=22–26 |doi=10.1016/S0040-4039(01)99487-0}}</ref> | |||
| Cairns-Smith is a staunch critic of other models of chemical evolution.<ref>{{cite book |author=Cairns-Smith, A. G. |title=Genetic takeover and the mineral origins of life |publisher=Cambridge University Press |location=Cambridge, UK |year=1982 |isbn=0-521-23312-7 }}</ref> However, he admits, that like many models of the origin of life, his own also has its shortcomings (Horgan 1991). | |||
| ==== Nucleobases ==== | |||
| In 2007, Kahr and colleagues reported their experiments to examine the idea that crystals can act as a source of transferable information, using crystals of ]. "Mother" crystals with imperfections were cleaved and used as seeds to grow "daughter" crystals from solution. They then examined the distribution of imperfections in the crystal system and found that the imperfections in the mother crystals were indeed reproduced in the daughters. The daughter crystals had many additional imperfections. For a gene-like behavior the additional imperfections should be much less than the parent ones, thus Kahr concludes that the crystals "were not faithful enough to store and transfer information form one generation to the next".<ref>{{cite journal |author=Bullard T, Freudenthal J, Avagyan S, Kahr B|title=Test of Cairns-Smith's ''crystals-as-genes'' hypothesis |journal=Faraday Discuss. |volume=136 |pages=231–45 |year=2007 |doi=10.1039/b616612c |url=http://www.rsc.org/publishing/journals/FD/article.asp?doi=b616612c}}</ref><ref>{{cite news|author=Caroline Moore|title=Crystals as genes?|date=16 July 2007|publisher=Chemical Science|url=http://www.rsc.org/Publishing/ChemScience/Volume/2007/08/Crystals_as_genes.asp }}</ref> | |||
| Nucleobases, such as guanine and adenine, can be synthesized from simple carbon and nitrogen sources, such as ] (HCN) and ammonia.<ref>{{cite journal |last=Oró |first=Joan |author-link=Joan Oró |date=16 September 1961 |title=Mechanism of Synthesis of Adenine from Hydrogen Cyanide under Possible Primitive Earth Conditions |journal=] |volume=191 |issue=4794 |pages=1193–1194 |bibcode=1961Natur.191.1193O |doi=10.1038/1911193a0 |pmid=13731264 |s2cid=4276712}}</ref> ] produces all four ribonucleotides when warmed with terrestrial minerals. Formamide is ubiquitous in the Universe, produced by the reaction of water and HCN. It can be concentrated by the evaporation of water.<ref name="Saladino2012">{{cite journal |last1=Saladino |first1=Raffaele |last2=Crestini |first2=Claudia |last3=Pino |first3=Samanta |last4=Costanzo |first4=Giovanna |last5=Di Mauro |first5=Ernesto |display-authors=3 |date=March 2012 |title=Formamide and the origin of life. |journal=] |volume=9 |issue=1 |pages=84–104 |bibcode=2012PhLRv...9...84S |doi=10.1016/j.plrev.2011.12.002 |pmid=22196896 |hdl=2108/85168 |url=https://art.torvergata.it/bitstream/2108/85168/1/PoLRev%202012.pdf |hdl-access=free |access-date=29 August 2019 |archive-date=27 January 2023 |archive-url=https://web.archive.org/web/20230127162359/https://art.torvergata.it/bitstream/2108/85168/1/PoLRev%202012.pdf |url-status=live }}</ref><ref name="Saladino2012b">{{cite journal |last1=Saladino |first1=Raffaele |last2=Botta |first2=Giorgia |last3=Pino |first3=Samanta |last4=Costanzo |first4=Giovanna |last5=Di Mauro |first5=Ernesto |display-authors=3 |date=July 2012 |title=From the one-carbon amide formamide to RNA all the steps are prebiotically possible |journal=] |volume=94 |issue=7 |pages=1451–1456 |doi=10.1016/j.biochi.2012.02.018 |pmid=22738728 |hdl=11573/515604}}</ref> HCN is poisonous only to ]s (]s and aerobic bacteria), which did not yet exist. It can play roles in other chemical processes such as the synthesis of the amino acid glycine.<ref name="Follmann2009"/> | |||
| ===Gold's "Deep-hot biosphere" model=== | |||
| In the 1970s, ] proposed the theory that life first developed not on the surface of the Earth, but several kilometers below the surface. The discovery in the late 1990s of ]s (filamental structures that are smaller than bacteria, but that may contain DNA) in deep rocks <ref name="nanobe"> microscopy-uk.org, Retrieved on ]</ref> might be seen as lending support to Gold's theory. | |||
| DNA and RNA components including uracil, cytosine and thymine can be synthesized under outer space conditions, using starting chemicals such as pyrimidine found in meteorites. Pyrimidine may have been formed in ] stars or in interstellar dust and gas clouds.<ref name="NASA-20150303">{{cite web |url=http://www.nasa.gov/content/nasa-ames-reproduces-the-building-blocks-of-life-in-laboratory |title=NASA Ames Reproduces the Building Blocks of Life in Laboratory |editor-last=Marlaire |editor-first=Ruth |date=3 March 2015 |work=Ames Research Center |publisher=NASA |access-date=5 March 2015 |url-status=live |archive-url=https://web.archive.org/web/20150305083306/http://www.nasa.gov/content/nasa-ames-reproduces-the-building-blocks-of-life-in-laboratory/ |archive-date=5 March 2015}}</ref> All four RNA-bases may be synthesized from formamide in high-energy density events like extraterrestrial impacts.<ref>{{cite journal |last1=Ferus |first1=Martin |last2=Nesvorný |first2=David |last3=Šponer |first3=Jiří |last4=Kubelík |first4=Petr |last5=Michalčíková |first5=Regina |last6=Shestivská |first6=Violetta |last7=Šponer |first7=Judit E. |last8=Civiš |first8=Svatopluk |display-authors=3 |year=2015 |title=High-energy chemistry of formamide: A unified mechanism of nucleobase formation |journal=] |volume=112 |issue=3 |pages=657–662 |doi=10.1073/pnas.1412072111 |pmid=25489115 |bibcode=2015PNAS..112..657F |pmc=4311869 |doi-access=free}}</ref> | |||
| It is now reasonably well established that ] life is plentiful at shallow depths in the Earth, up to {{convert|5|km|mi}} below the surface,<ref name="nanobe"/> in the form of ], rather than the better-known ] (which live in more accessible conditions). It is claimed that discovery of microbial life below the surface of another body in our ] would lend significant credence to this theory. ] also asserted that a trickle of food from a deep, unreachable, source is needed for survival because life arising in a puddle of organic material is likely to consume all of its food and become extinct. Gold's theory is that flow of food is due to out-gassing of primordial ] from the ]; more conventional explanations of the food supply of deep microbes (away from sedimentary carbon compounds) is that the organisms subsist on ] released by an interaction between water and (reduced) iron compounds in rocks. | |||
| Other pathways for synthesizing bases from inorganic materials have been reported.<ref name="Basile1984">{{cite journal |last1=Basile |first1=Brenda |last2=Lazcano |first2=Antonio |author2-link=Antonio Lazcano |last3=Oró |first3=Joan |year=1984 |title=Prebiotic syntheses of purines and pyrimidines |journal=] |volume=4 |issue=12 |pages=125–131 |bibcode=1984AdSpR...4l.125B |doi=10.1016/0273-1177(84)90554-4 |pmid=11537766}}</ref> Freezing temperatures are advantageous for the synthesis of purines, due to the concentrating effect for key precursors such as hydrogen cyanide.<ref>{{cite journal |last=Orgel |first=Leslie E. |date=August 2004 |title=Prebiotic Adenine Revisited: Eutectics and Photochemistry |journal=] |volume=34 |issue=4 |pages=361–369 |bibcode=2004OLEB...34..361O |doi=10.1023/B:ORIG.0000029882.52156.c2 |pmid=15279171 |s2cid=4998122}}</ref> However, while adenine and guanine require freezing conditions for synthesis, cytosine and uracil may require boiling temperatures.<ref>{{cite journal |last1=Robertson |first1=Michael P. |last2=Miller |first2=Stanley L. |date=29 June 1995 |title=An efficient prebiotic synthesis of cytosine and uracil |journal=Nature |volume=375 |issue=6534 |pages=772–774 |bibcode=1995Natur.375..772R |doi=10.1038/375772a0 |pmid=7596408 |s2cid=4351012}}</ref> Seven amino acids and eleven types of nucleobases formed in ice when ammonia and ] were left in a freezer for 25 years.<ref>{{cite journal |last=Fox |first=Douglas |date=1 February 2008 |title=Did Life Evolve in Ice? |journal=] |url=https://www.discovermagazine.com/planet-earth/did-life-evolve-in-ice |access-date=2008-07-03 |url-status=live |archive-url=https://web.archive.org/web/20080630043228/http://discovermagazine.com/2008/feb/did-life-evolve-in-ice |archive-date=30 June 2008}}</ref><ref>{{cite journal |last1=Levy |first1=Matthew |last2=Miller |first2=Stanley L. |last3=Brinton |first3=Karen |last4=Bada |first4=Jeffrey L. |author4-link=Jeffrey L. Bada |date=June 2000 |title=Prebiotic Synthesis of Adenine and Amino Acids Under Europa-like Conditions |journal=] |volume=145 |issue=2 |pages=609–613 |bibcode=2000Icar..145..609L |doi=10.1006/icar.2000.6365 |pmid=11543508}}</ref> S-]s (alternative nucleobases), pyrimidines including cytosine and uracil, and adenine can be synthesized by subjecting a urea solution to freeze-thaw cycles under a reductive atmosphere, with spark discharges as an energy source.<ref>{{cite journal |last1=Menor-Salván |first1=César |last2=Ruiz-Bermejo |first2=Marta |last3=Guzmán |first3=Marcelo I. |last4=Osuna-Esteban |first4=Susana |last5=Veintemillas-Verdaguer |first5=Sabino |display-authors=3 |date=20 April 2009 |title=Synthesis of Pyrimidines and Triazines in Ice: Implications for the Prebiotic Chemistry of Nucleobases |journal=] |volume=15 |issue=17 |pages=4411–4418 |doi=10.1002/chem.200802656 |pmid=19288488}}</ref> The explanation given for the unusual speed of these reactions at such a low temperature is ], which crowds impurities in microscopic pockets of liquid within the ice, causing the molecules to collide more often.<ref>{{cite journal |last1=Roy |first1=Debjani |last2=Najafian |first2=Katayoun |last3=von Ragué Schleyer |first3=Paul |author-link3=Paul von Ragué Schleyer |date=30 October 2007 |title=Chemical evolution: The mechanism of the formation of adenine under prebiotic conditions |journal=] |volume=104 |issue=44 |pages=17272–17277 |bibcode=2007PNAS..10417272R |doi=10.1073/pnas.0708434104 |pmc=2077245 |pmid=17951429 |doi-access=free}}</ref> | |||
| ==="Primitive" extraterrestrial life=== | |||
| An alternative to Earthly abiogenesis is the hypothesis that primitive life may have originally formed extraterrestrially, either in space or on a nearby planet (Mars). (Note that exogenesis is related to, but not the same as, the notion of ]). A supporter of this theory was ]. | |||
| ==== Peptides ==== | |||
| Organic compounds are relatively common in space, especially in the outer solar system where volatiles are not evaporated by solar heating. ]s are encrusted by outer layers of dark material, thought to be a ]-like substance composed of complex organic material formed from simple carbon compounds after reactions initiated mostly by irradiation by ] light. It is supposed that a rain of material from ]s could have brought significant quantities of such complex organic molecules to Earth. | |||
| Prebiotic peptide synthesis is proposed to have occurred through a number of possible routes. Some center on high temperature/concentration conditions in which condensation becomes energetically favorable, while others focus on the availability of plausible prebiotic condensing agents.<ref name=":0">{{Cite journal |last1=Frenkel-Pinter |first1=Moran |last2=Samanta |first2=Mousumi |last3=Ashkenasy |first3=Gonen |last4=Leman |first4=Luke J. |date=2020-06-10 |title=Prebiotic Peptides: Molecular Hubs in the Origin of Life |url=https://pubs.acs.org/doi/10.1021/acs.chemrev.9b00664 |journal=Chemical Reviews |volume=120 |issue=11 |pages=4707–4765 |doi=10.1021/acs.chemrev.9b00664 |pmid=32101414 |bibcode=2020ChRv..120.4707F |issn=0009-2665}}</ref>{{Explain|date=April 2024}} | |||
| Experimental evidence for the formation of peptides in uniquely concentrated environments is bolstered by work suggesting that wet-dry cycles and the presence of specific salts can greatly increase spontaneous condensation of glycine into poly-glycine chains.<ref>{{Cite journal |title=Prebiotic condensation through wet–dry cycling regulated by deliquescence |doi=10.1038/s41467-019-11834-1 |pmc=6778215 |pmid=31586058 | date=2019 | last1=Campbell | first1=Thomas D. | last2=Febrian | first2=Rio | last3=McCarthy | first3=Jack T. | last4=Kleinschmidt | first4=Holly E. | last5=Forsythe | first5=Jay G. | last6=Bracher | first6=Paul J. | journal=Nature Communications | volume=10 | issue=1 | page=4508 |bibcode=2019NatCo..10.4508C }}</ref> Other work suggests that while mineral surfaces, such as those of pyrite, calcite, and rutile catalyze peptide condensation, they also catalyze their hydrolysis. The authors suggest that additional chemical activation or coupling would be necessary to produce peptides at sufficient concentrations. Thus, mineral surface catalysis, while important, is not sufficient alone for peptide synthesis.<ref>{{Cite journal |last1=Marshall-Bowman |first1=Karina |last2=Ohara |first2=Shohei |last3=Sverjensky |first3=Dimitri A. |last4=Hazen |first4=Robert M. |last5=Cleaves |first5=H. James |date=October 2010 |title=Catalytic peptide hydrolysis by mineral surface: Implications for prebiotic chemistry |journal=Geochimica et Cosmochimica Acta |volume=74 |issue=20 |pages=5852–5861 |doi=10.1016/j.gca.2010.07.009 |bibcode=2010GeCoA..74.5852M |issn=0016-7037}}</ref> | |||
| An alternative but related hypothesis, proposed to explain the presence of life on Earth so soon after the planet had cooled down, with apparently very little time for prebiotic evolution, is that life formed first on early ]. Due to its smaller size Mars cooled before Earth (a difference of hundreds of millions of years), allowing prebiotic processes there while Earth was still too hot. Life was then transported to the cooled Earth when crustal material was blasted off Mars by asteroid and comet impacts. Mars continued to cool faster and eventually became hostile to the continued evolution or even existence of life (it lost its atmosphere due to low volcanism); Earth is following the same fate as Mars, but at a slower rate. | |||
| Many prebiotically plausible condensing/activating agents have been identified, including the following: cyanamide, dicyanamide, dicyandiamide, diaminomaleonitrile, urea, trimetaphosphate, NaCl, CuCl<sub>2,</sub> (Ni,Fe)S, CO, carbonyl sulfide (COS), carbon disulfide (CS<sub>2</sub>)<sub>,</sub> SO<sub>2,</sub> and diammonium phosphate (DAP).<ref name=":0" /> | |||
| Neither hypothesis actually answers the question of how life first originated, but merely shifts it to another planet or a comet. However, the advantage of an extraterrestrial origin of primitive life is that life is not required to have evolved on each planet it occurs on, but rather in a single location, and then spread about the galaxy to other star systems via cometary and/or meteorite impact. Evidence to support the plausibility of the concept is scant, but it finds support in recent study of Martian meteorites found in Antarctica and in studies of ] microbes.<ref></ref> Additional support comes from a recent discovery of a bacterial ecosytem whose energy source is radioactivity.<ref>{{cite journal | |||
| |title = Long-Term Sustainability of a High-Energy, Low-Diversity Crustal Biome | |||
| |first = Li-Hung | |||
| |last = Lin | |||
| |coauthors = Pei-Ling Wang, Douglas Rumble, Johanna Lippmann-Pipke, Erik Boice, Lisa M. Pratt, Barbara Sherwood Lollar, Eoin L. Brodie, Terry C. Hazen, Gary L. Andersen, Todd Z. DeSantis, Duane P. Moser, Dave Kershaw, T. C. Onstott | |||
| |journal = Science | |||
| |month = October | |||
| |year = 2006 | |||
| |volume = 314 | |||
| |pages = 479–482 | |||
| |id = 5798 | |||
| |doi = 10.1126/science.1127376 | |||
| |pmid = 17053150 | |||
| }}</ref> | |||
| An experiment reported in 2024 used a sapphire substrate with a web of thin cracks under a heat flow, similar to the environment of ], as a mechanism to separate and concentrate prebiotically relevant building blocks from a dilute mixture, purifying their concentration by up to three orders of magnitude. The authors propose this as a plausible model for the origin of complex biopolymers.<ref>{{Cite journal |last1=Matreux |first1=Thomas |last2=Aikkila |first2=Paula |last3=Scheu |first3=Bettina |last4=Braun |first4=Dieter |last5=Mast |first5=Christof B. |date=April 2024 |title=Heat flows enrich prebiotic building blocks and enhance their reactivity |journal=Nature |volume=628 |issue=8006 |pages=110–116 |doi=10.1038/s41586-024-07193-7 |issn=1476-4687 |pmc=10990939 |pmid=38570715|bibcode=2024Natur.628..110M }}</ref> This presents another physical process that allows for concentrated peptide precursors to combine in the right conditions. A similar role of increasing amino acid concentration has been suggested for clays as well.<ref>{{Cite journal |last=Paecht-Horowitz |first=Mella |date=1974-01-01 |title=The possible role of clays in prebiotic peptide synthesis |journal=Origins of Life |volume=5 |issue=1 |pages=173–187 |doi=10.1007/BF00927022 |pmid=4842069 |bibcode=1974OrLi....5..173P |issn=1573-0875}}</ref> | |||
| A recent experiment led by Jason Dworkin, subjected a frozen mixture of water, ], ] and ] to ], mimicking conditions found in an extraterrestrial environment. This combination yielded large amounts of organic material that self-organised to form bubbles when immersed in water. Dworkin considered these bubbles to resemble cell membranes that enclose and concentrate the chemistry of life, separating their interior from the outside world. | |||
| While all of these scenarios involve the condensation of amino acids, the prebiotic synthesis of peptides from simpler molecules such as CO, NH<sub>3</sub> and C, skipping the step of amino acid formation, is very efficient.<ref>{{Cite journal |last1=Krasnokutski |first1=S. A. |last2=Chuang |first2=K.-J. |last3=Jäger |first3=C. |last4=Ueberschaar |first4=N. |last5=Henning |first5=Th. |date=2022-02-10 |title=A pathway to peptides in space through the condensation of atomic carbon |url=https://www.nature.com/articles/s41550-021-01577-9 |journal=Nature Astronomy |language=en |volume=6 |issue=3 |pages=381–386 |doi=10.1038/s41550-021-01577-9 |arxiv=2202.12170 |bibcode=2022NatAs...6..381K |issn=2397-3366}}</ref><ref>{{Cite journal |last1=Krasnokutski |first1=Serge A. |last2=Jäger |first2=Cornelia |last3=Henning |first3=Thomas |last4=Geffroy |first4=Claude |last5=Remaury |first5=Quentin B. |last6=Poinot |first6=Pauline |date=2024-04-19 |title=Formation of extraterrestrial peptides and their derivatives |journal=Science Advances |language=en |volume=10 |issue=16 |pages=eadj7179 |doi=10.1126/sciadv.adj7179 |issn=2375-2548 |pmc=11023503 |pmid=38630826|arxiv=2405.00744 |bibcode=2024SciA...10J7179K }}</ref> | |||
| The bubbles produced in these experiments were between {{convert|10|to|40|µm|in}}, or about the size of red blood cells. Remarkably, the bubbles ], or glowed, when exposed to ]. Absorbing UV and converting it into visible light in this way was considered one possible way of providing energy to a primitive cell. If such bubbles played a role in the origin of life, the fluorescence could have been a precursor to primitive ]. Such fluorescence also provides the benefit of acting as a sunscreen, diffusing any damage that otherwise would be inflicted by UV radiation. Such a protective function would have been vital for life on the early Earth, since the ], which blocks out the sun's most destructive UV rays, did not form until after photosynthetic life began to produce oxygen.<ref name=StarStuff>{{cite journal |author=Mullen L |title=Building Life from Star-Stuff |journal=Astrobiology Magazine |date=September 05, 2005 |url=http://www.astrobio.net/news/article1702.html}}</ref> | |||
| == Producing suitable vesicles == | |||
| ===Lipid World=== | |||
| This theory postulates that the first self-replicating object was lipid-like.<ref></ref> It is known that phospholipids form bilayers in water while under agitation– the same structure as in cell membranes. These molecules were not present on early earth, however other amphiphilic long chain molecules also form membranes. Furthermore, these bodies may expand (by insertion of additional lipids), and under excessive expansion may undergo spontaneous splitting which preserves the same size and composition of lipids in the two progenies. The main idea in this theory is that the molecular composition of the lipid bodies is the preliminary way for information storage, and evolution led to the appearance of polymer entities such as RNA or DNA that may store information favorably. Still, no biochemical mechanism has been offered to support the Lipid World theory. | |||
| {{further|Gard model|Self-organization#Biology|Cellularization}} | |||
| ===Polyphosphates=== | |||
| The problem with most scenarios of abiogenesis is that the thermodynamic equilibrium of amino acid versus peptides is in the direction of separate amino acids. What has been missing is some force that drives polymerization. The resolution of this problem may well be in the properties of ]s.<ref>{{cite journal |author=Brown MR, Kornberg A |title=Inorganic polyphosphate in the origin and survival of species |journal=Proc. Natl. Acad. Sci. U.S.A. |volume=101 |issue=46 |pages=16085–7 |year=2004 |month=November |pmid=15520374 |pmc=528972 |doi=10.1073/pnas.0406909101 |url=http://www.pnas.org/cgi/pmidlookup?view=long&pmid=15520374}}</ref><ref></ref> Polyphosphates are formed by polymerization of ordinary monophosphate ions PO<sub>4</sub><sup>−3</sup>. Several mechanisms for such polymerization have been suggested. Polyphosphates cause polymerization of amino acids into peptides {{Fact|date=April 2009}}. They are also logical precursors in the synthesis of such key biochemical compounds as ATP. A key issue seems to be that calcium reacts with soluble phosphate to form insoluble ] (]), so some plausible mechanism must be found to keep calcium ions from causing precipitation of phosphate. | |||
| There has been much work on this topic over the years, but an interesting new idea is that meteorites may have introduced reactive phosphorus species on the early earth.<ref>{{cite journal |author=Pasek MA |title=Rethinking early Earth phosphorus geochemistry |journal=Proc. Natl. Acad. Sci. U.S.A. |volume=105 |issue=3 |pages=853–8 |year=2008 |month=January |pmid=18195373 |pmc=2242691 |doi=10.1073/pnas.0708205105 |url=http://www.pnas.org/cgi/pmidlookup?view=long&pmid=18195373}}</ref> | |||
| ]s form spontaneously by ] in solution: the ] (a closed bilayer), the ] and the bilayer.]] | |||
| ===PAH world hypothesis=== | |||
| {{Main|PAH world hypothesis}} | |||
| Other sources of complex molecules have been postulated, including extraterrestrial stellar or interstellar origin. For example, from spectral analyses, organic molecules are known to be present in comets and meteorites. In 2004, a team detected traces of ] (PAH's) in a ].<ref>{{cite journal |author=Witt AN, Vijh UP, Gordon KD |title=Discovery of Blue Fluorescence by Polycyclic Aromatic Hydrocarbon Molecules in the Red Rectangle |journal=Bulletin of the American Astronomical Society |volume=35 |pages=1381 |year=2003 |url=http://web.archive.org/web/20031219175322/http://www.aas.org/publications/baas/v35n5/aas203/189.htm}}</ref> Those are the most complex molecules so far found in space. The use of PAH's has also been proposed as a precursor to the RNA world in the ].<ref>Battersby, S. (2004). Space molecules point to organic origins. Retrieved January 11, 2004 from http://www.newscientist.com/article/dn4552-space-molecules-point-to-organic-origins.html</ref> The ] has recently detected a star, HH 46-IR, which is forming by a process similar to that by which the sun formed. In the disk of material surrounding the star, there is a very large range of molecules, including cyanide compounds, hydrocarbons, and carbon monoxide. PAHs have also been found all over the surface of galaxy M81, which is 12 million light years away from the Earth, confirming their widespread distribution in space.<ref>Astrobiology Mgazine Accessed 26 April 2008</ref> | |||
| The largest unanswered question in evolution is how simple protocells first arose and differed in reproductive contribution to the following generation, thus initiating the evolution of life. The ] theory postulates that the first self-replicating object was ]-like.<ref>{{cite web |url=http://www.weizmann.ac.il/molgen/Lancet/research/prebiotic-evolution |title=Systems Prebiology-Studies of the origin of Life |last=Lancet |first=Doron |date=30 December 2014 |website=The Lancet Lab |publisher=Department of Molecular Genetics; ] |location=Rehovot, Israel |access-date=26 June 2015 |url-status=live |archive-url=https://web.archive.org/web/20150626180507/http://www.weizmann.ac.il/molgen/Lancet/research/prebiotic-evolution |archive-date=26 June 2015}}</ref><ref>{{cite journal |last1=Segré |first1=Daniel |last2=Ben-Eli |first2=Dafna |last3=Deamer |first3=David W. |last4=Lancet |first4=Doron |date=February 2001 |title=The Lipid World |url=http://www.weizmann.ac.il/molgen/Lancet/sites/molgen.Lancet/files/uploads/segre_lipid_world.pdf |journal=] |volume=31 |issue=1–2 |pages=119–145 |doi=10.1023/A:1006746807104 |pmid=11296516 |bibcode=2001OLEB...31..119S |s2cid=10959497 |url-status=live |archive-url=https://web.archive.org/web/20150626225745/http://www.weizmann.ac.il/molgen/Lancet/sites/molgen.Lancet/files/uploads/segre_lipid_world.pdf |archive-date=26 June 2015}}</ref> Phospholipids form ]s in water while under agitation—the same structure as in cell membranes. These molecules were not present on early Earth, but other ] long-chain molecules also form membranes. These bodies may expand by insertion of additional lipids, and may spontaneously split into two ] of similar size and composition. Lipid bodies may have provided sheltering envelopes for information storage, allowing the evolution and preservation of polymers like RNA that store information. Only one or two types of amphiphiles have been studied which might have led to the development of vesicles.<ref name="Chen 2010"/> There is an enormous number of possible arrangements of lipid bilayer membranes, and those with the best reproductive characteristics would have converged toward a hypercycle reaction,<ref>{{cite journal |last1=Eigen |first1=Manfred |author-link1=Manfred Eigen |last2=Schuster |first2=Peter |author-link2=Peter Schuster |date=November 1977 |title=The Hypercycle. A Principle of Natural Self-Organization. Part A: Emergence of the Hypercycle |url=http://jaguar.biologie.hu-berlin.de/~wolfram/pages/seminar_theoretische_biologie_2007/literatur/schaber/Eigen1977Naturwissenschaften64.pdf |journal=] |volume=64 |issue=11 |pages=541–65 |bibcode=1977NW.....64..541E |doi=10.1007/bf00450633 |pmid=593400 |s2cid=42131267 |archive-url=https://web.archive.org/web/20160303194728/http://jaguar.biologie.hu-berlin.de/~wolfram/pages/seminar_theoretische_biologie_2007/literatur/schaber/Eigen1977Naturwissenschaften64.pdf |archive-date=3 March 2016 |ref=none}} | |||
| ===Multiple genesis=== | |||
| Different forms of life may have appeared quasi-simultaneously in the early history of Earth.<ref>'''' Scientific American. 19 November 2007</ref> The other forms may be extinct, leaving distinctive fossils through their different biochemistry (e.g., ]), survive as ], or simply be unnoticed through their being ] to organisms of the current life tree. Hartman<ref>Hartman, Hyman (1998) "Photosynthesis and the Origin of Life" (Origins of Life and Evolution of Biospheres, Volume 28, Numbers 4–6 / October, 1998)</ref> for example combines a number of theories together, by proposing that: | |||
| <blockquote>The first organisms were self-replicating iron-rich clays which fixed carbon dioxide into oxalic and other dicarboxylic acids. This system of replicating clays and their metabolic phenotype then evolved into the sulfide rich region of the hotspring acquiring the ability to fix nitrogen. Finally phosphate was incorporated into the evolving system which allowed the synthesis of nucleotides and phospholipids. If biosynthesis recapitulates biopoesis, then the synthesis of amino acids preceded the synthesis of the purine and pyrimidine bases. Furthermore the polymerization of the amino acid thioesters into polypeptides preceded the directed polymerization of amino acid esters by polynucleotides.</blockquote> | |||
| ]'s ] suggests that multiple forms of bacteria entered into symbiotic relationship to form the eucaryotic cell. The horizontal transfer of genetic material between bacteria promotes such symbiotic relationships, and thus many separate organisms may have contributed to building what has been recognised as the ] (LUCA) of modern organisms. ]'s ], proposes that such bacterial symbiosis establishes the environment as a system produced by and supportive of life. His arguments strongly weaken the case for life having evolved elsewhere in the solar system. | |||
| * {{cite journal |last1=Eigen |first1=Manfred |author1-link=Manfred Eigen |last2=Schuster |first2=Peter |author2-link=Peter Schuster |year=1978 |title=The Hypercycle. A Principle of Natural Self-Organization. Part B: The Abstract Hypercycle |url=http://jaguar.biologie.hu-berlin.de/~wolfram/pages/seminar_theoretische_biologie_2007/literatur/schaber/Eigen1978Naturwissenschaften65a.pdf |journal=] |volume=65 |issue=1 |pages=7–41 |bibcode=1978NW.....65....7E |doi=10.1007/bf00420631 |s2cid=1812273 |archive-url=https://web.archive.org/web/20160303203325/http://jaguar.biologie.hu-berlin.de/~wolfram/pages/seminar_theoretische_biologie_2007/literatur/schaber/Eigen1978Naturwissenschaften65a.pdf |archive-date=3 March 2016 |ref=none}} | |||
| ==References== | |||
| * {{cite journal |last1=Eigen |first1=Manfred |author1-link=Manfred Eigen |last2=Schuster |first2=Peter |author-link2=Peter Schuster |date=July 1978 |title=The Hypercycle. A Principle of Natural Self-Organization. Part C: The Realistic Hypercycle |url=http://jaguar.biologie.hu-berlin.de/~wolfram/pages/seminar_theoretische_biologie_2007/literatur/schaber/Eigen1978Naturwissenschaften65b.pdf |journal=] |volume=65 |issue=7 |pages=341–369 |bibcode=1978NW.....65..341E |doi=10.1007/bf00439699 |s2cid=13825356 |archive-url=https://web.archive.org/web/20160616180402/http://jaguar.biologie.hu-berlin.de/~wolfram/pages/seminar_theoretische_biologie_2007/literatur/schaber/Eigen1978Naturwissenschaften65b.pdf |archive-date=16 June 2016 |ref=none}}</ref><ref>{{cite journal |last1=Markovitch |first1=Omer |last2=Lancet |first2=Doron |date=Summer 2012 |title=Excess Mutual Catalysis Is Required for Effective Evolvability |journal=] |volume=18 |issue=3 |pages=243–266 |doi=10.1162/artl_a_00064 |pmid=22662913 |s2cid=5236043 |doi-access=free}}</ref> a positive ] composed of two mutual catalysts represented by a membrane site and a specific compound trapped in the vesicle. Such site/compound pairs are transmissible to the daughter vesicles leading to the emergence of distinct ] of vesicles, which would have allowed ].<ref>{{cite journal |last=Tessera |first=Marc |year=2011 |title=Origin of Evolution ''versus'' Origin of Life: A Shift of Paradigm |journal=] |volume=12 |issue=6 |pages=3445–3458 |doi=10.3390/ijms12063445 |pmc=3131571 |pmid=21747687 |doi-access=free}} Special Issue: "Origin of Life 2011"</ref> | |||
| {{reflist|2}} | |||
| A ] is a self-organized, self-ordered, spherical collection of lipids proposed as a stepping-stone to the origin of life.<ref name="Chen 2010">{{cite journal |first1=Irene A. |last1=Chen |first2= Peter |last2=Walde |title=From Self-Assembled Vesicles to Protocells |journal=] |date=July 2010 |volume=2 |issue=7 |page=a002170 |doi= 10.1101/cshperspect.a002170 |pmc=2890201 |pmid=20519344}}</ref> A functional protocell has (as of 2014) not yet been achieved in a laboratory setting.<ref name="Exploring">{{cite web |title=Protocells |url=http://exploringorigins.org/protocells.html |url-status=live |archive-url=https://web.archive.org/web/20140228083459/http://exploringorigins.org/protocells.html |archive-date=28 February 2014 |access-date=September 13, 2023 |website=Exploring Life's Origins: A Virtual Exhibit |publisher=] |location=Arlington County, Virginia}}</ref><ref name="Chen 2006">{{cite journal |last=Chen |first=Irene A. |date=8 December 2006 |title=The Emergence of Cells During the Origin of Life |journal=] |volume=314 |issue=5805 |pages=1558–1559 |doi=10.1126/science.1137541 |pmid=17158315 |doi-access=free}}</ref><ref name="Discover 2004">{{cite magazine |last=Zimmer |first=Carl |author-link=Carl Zimmer |date=26 June 2004 |title=What Came Before DNA? |url=https://www.discovermagazine.com/planet-earth/what-came-before-dna |url-status=live |archive-url=https://web.archive.org/web/20140319001351/http://discovermagazine.com/2004/jun/cover |archive-date=19 March 2014 |journal=]}}</ref> Self-assembled ] are essential components of primitive cells.<ref name="Chen 2010" /> The theory of classical irreversible thermodynamics treats self-assembly under a generalized chemical potential within the framework of ]s.<ref>{{cite journal |last=Onsager |first=Lars |date=1931 |title=Reciprocal Relations in Irreversible Processes I |journal=Physical Review |volume=37 |issue=4 |page=405 |doi= 10.1103/PhysRev.37.405 |bibcode=1931PhRv...37..405O|doi-access=free }}</ref><ref>{{cite journal |last=Onsager |first=Lars |date=1931 |title=Reciprocal Relations in Irreversible Processes II |journal= Physical Review |volume=38 |issue=12 |page=2265 |doi=10.1103/PhysRev.38.2265|bibcode=1931PhRv...38.2265O |doi-access=free }}</ref><ref>{{cite book |last=Prigogine |first=Ilya |author-link=Ilya Prigogine |year=1967 |title=An Introduction to the Thermodynamics of Irreversible Processes |publisher=] |location=New York}}</ref> The ] requires that overall ] increases, yet life is distinguished by its great degree of organization. Therefore, a boundary is needed to separate ordered ] from chaotic non-living matter.<ref name="SciAm 2007">{{cite journal |last=Shapiro |first=Robert |author-link=Robert Shapiro (chemist) |date=June 2007 |title=A Simpler Origin for Life |url=http://www.scientificamerican.com/article/a-simpler-origin-for-life/ |url-status=live |journal=] |volume=296 |issue=6 |pages=46–53 |bibcode=2007SciAm.296f..46S |doi=10.1038/scientificamerican0607-46 |pmid=17663224 |archive-url=https://web.archive.org/web/20150614000643/http://www.scientificamerican.com/article/a-simpler-origin-for-life/ |archive-date=14 June 2015}}</ref> | |||
| ==Further reading== | |||
| * {{cite journal |last=Arrhenius |first=Gustaf |authorlink= |coauthors=''et al.'' |year=1997 |month= |title=Entropy and Charge in Molecular Evolution—the Case of Phosphate |journal=Journal of Theoretical Biology |volume=187 |issue=4 |pages=503–522 |doi=10.1006/jtbi.1996.0385 }} | |||
| * Buehler, Lukas K. (2000–2005) ''The physico-chemical basis of life'', http://www.whatislife.com/about.html accessed 27 October 2005. | |||
| * {{cite book| | |||
| title=The Fifth Miracle| | |||
| last=Davies| | |||
| first=Paul| | |||
| authorlink=Paul Davies| | |||
| year=1998| | |||
| publisher=Penguin Science, London| | |||
| isbn=0-140-28226-2 | |||
| }} | |||
| * {{cite book| | |||
| title=Vital Dust: The Origin and Evolution of Life on Earth| | |||
| last=De Duve | | |||
| first=Christian| | |||
| authorlink=Christian de Duve| | |||
| year=1996| | |||
| month=January| | |||
| publisher=]| | |||
| isbn=0-465-09045-1 | |||
| }} | |||
| * {{cite journal | author=Fernando CT, Rowe, J| title=Natural selection in chemical evolution | journal=Journal of Theoretical Biology | year=2007 | volume=247 | pages=152–67| doi=10.1016/j.jtbi.2007.01.028}} | |||
| * {{cite journal |last=Hartman |first=Hyman |authorlink= |coauthors= |year=1998 |month= |title=Photosynthesis and the Origin of Life |journal=Origins of Life and Evolution of Biospheres |volume=28 |issue=4–6 |pages=515–521 |doi=10.1023/A:1006548904157 }} | |||
| * {{cite book |title=Things come to life. Spontaneous generation revisited |last=Harris |first=Henry |authorlink= |coauthors= |year=2002 |publisher=Oxford University Press |location=Oxford |isbn=0198515383 |pages= }} | |||
| * {{cite book| | |||
| last=Hazen| | |||
| first=Robert M.| | |||
| publisher=Joseph Henry Press| | |||
| isbn=0-309-09432-1| | |||
| year=2005| | |||
| month=December| | |||
| title=Genesis: The Scientific Quest for Life's Origins| | |||
| url=http://newton.nap.edu/books/0309094321/html | |||
| }} | |||
| * {{cite book| | |||
| title=The Case of the Missing Neutrino's and other Curious Phenomena of the Universe| | |||
| last=Gribbon | | |||
| first=John| | |||
| year=1998| | |||
| publisher=Penguin Science, London| | |||
| isbn=0-140-28734-5 | |||
| }} | |||
| * {{cite journal| author=Horgan, J |title=In the beginning |journal=]| year=1991 |volume=264 | pages=100–109}} (Cited on p. 108). | |||
| * {{cite journal| author=Huber, C. and Wächterhäuser, G., |title=Peptides by activation of amino acids with CO on (Ni,Fe)S surfaces: implications for the origin of life|journal=]| year=1998 |volume=281 | pages=670–672 |doi=10.1126/science.281.5377.670 |pmid=9685253}} (Cited on p. 108). | |||
| * {{cite book |title= Life on a Young Planet: The First Three Billion Years of Evolution on Earth |last=Knoll |first=Andrew H. |year=2003 |publisher= Princeton University Press |location=}} | |||
| <!--*{{cite book| | |||
| last=Luisi| | |||
| first= Pier L.| | |||
| publisher=Cambridge University Press| | |||
| isbn=0-521-82117-7| | |||
| year=2006| | |||
| title=Emergence of Life: From Chemical Origins to Synthetic Biology| | |||
| url=http://www.cambridge.org/catalogue/catalogue.asp?isbn=9780521821179 | |||
| }}--> | |||
| * {{cite book |title= The Emergence of Life: From Chemical Origins to Synthetic Biology | |||
| |last=Luisi |first=Pier Luigi |year=2006 |publisher= Cambridge University Press |location= }} | |||
| * {{cite journal| author=Martin, W. and Russell M.J. |title=On the origins of cells: a hypothesis for the evolutionary transitions from abiotic geochemistry to chemoautotrophic prokaryotes, and from prokaryotes to nucleated cells | |||
| |journal=Philosophical Transactions of the Royal Society: Biological sciences| year=2002 |volume=358 | pages=59–85 |doi=10.1098/rstb.2002.1183}} | |||
| * {{cite book| | |||
| title=The Origins of Life: From the Birth of Life to the Origin of Language| | |||
| last=Maynard Smith| | |||
| first=John| | |||
| authorlink=John Maynard Smith| | |||
| coauthors=Szathmary, Eors|date=2000-03-16| | |||
| publisher=Oxford Paperbacks| | |||
| isbn=0-19-286209-X | |||
| }} | |||
| * Morowitz, Harold J. (1992) "Beginnings of Cellular Life: Metabolism Recapitulates Biogenesis". Yale University Press. ISBN 0-300-05483-1 | |||
| * NASA Astrobiology Institute: | |||
| * NASA Specialized Center of Research and Training in Exobiology: | |||
| * {{cite journal |last=Pitsch |first=Stefan |authorlink= |coauthors=Krishnamurthy, Ramanarayanan; Arrhenius, Gustaf |year=2000 |month= |title=Concentration of Simple Aldehydes by Sulfite-Containing Double-Layer Hydroxide Minerals: Implications for Biopoesis |journal=Helvetica Chimica Acta |volume=83 |issue=9 |pages=2398 2411 |doi= 10.1002/1522-2675(20000906)83:9<2398::AID-HLCA2398>3.0.CO;2-5|url=http://www3.interscience.wiley.com/cgi-bin/abstract/73501648/ABSTRACT |accessdate= |format=abstract }} | |||
| * {{cite journal | author=Russell MJ, Hall AJ, Cairns-Smith AG, Braterman PS | title=Submarine hot springs and the origin of life | journal=Nature | year=1988 | volume=336 | page=117 | doi=10.1038/336117a0 | pages=117}} | |||
| * | |||
| * | |||
| Irene Chen and ] suggest that elementary protocells can give rise to cellular behaviors including primitive forms of differential reproduction, competition, and energy storage.<ref name="Chen 2006" /> Competition for membrane molecules would favor stabilized membranes, suggesting a selective advantage for the evolution of cross-linked fatty acids and even the ]s of today.<ref name="Chen 2006" /> Such ] would allow for metabolism within the membrane and the exchange of small molecules, while retaining large biomolecules inside. Such a membrane is needed for a cell to create its own ] to store energy by pumping ions across the membrane.<ref>{{harvnb|Chang|2007}}</ref><ref name="Lane 2015" /> Fatty acid vesicles in conditions relevant to alkaline hydrothermal vents can be stabilized by isoprenoids which are synthesized by the formose reaction; the advantages and disadvantages of isoprenoids incorporated within the lipid bilayer in different microenvironments might have led to the divergence of the membranes of archaea and bacteria.<ref>{{Cite journal |last1=Jordan |first1=Sean F. |last2=Nee |first2=Eloise |last3=Lane |first3=Nick |author3-link=Nick Lane |date=2019-12-06 |title=Isoprenoids enhance the stability of fatty acid membranes at the emergence of life potentially leading to an early lipid divide |journal=Interface Focus |volume=9 |issue=6 |page=20190067 |doi=10.1098/rsfs.2019.0067 |pmc=6802135 |pmid=31641436}}</ref> | |||
| ==See also== | |||
| <div style="-moz-column-count:4; column-count:4;"> | |||
| Laboratory experiments have shown that vesicles can undergo an evolutionary process under pressure cycling conditions.<ref>{{Cite journal |last1=Mayer |first1=Christian |last2=Schreiber |first2=Ulrich |last3=Dávila |first3=María J. |last4=Schmitz |first4=Oliver J. |last5=Bronja |first5=Amela |last6=Meyer |first6=Martin |last7=Klein |first7=Julia |last8=Meckelmann |first8=Sven W. |date=2018 |title=Molecular Evolution in a Peptide-Vesicle System |journal=Life |language=en |volume=8 |issue=2 |pages=16 |doi=10.3390/life8020016 |doi-access=free |pmid=29795023 |pmc=6027363 |bibcode=2018Life....8...16M |issn=2075-1729}}</ref> Simulating the systemic environment in tectonic ] within the ], pressure cycling leads to the periodic formation of vesicles.<ref>{{Cite journal |last1=Mayer |first1=Christian |last2=Schreiber |first2=Ulrich |last3=Dávila |first3=María J. |date=2015-06-01 |title=Periodic Vesicle Formation in Tectonic Fault Zones—an Ideal Scenario for Molecular Evolution |journal=Origins of Life and Evolution of Biospheres |language=en |volume=45 |issue=1 |pages=139–148 |doi=10.1007/s11084-015-9411-z |issn=1573-0875 |pmc=4457167 |pmid=25716918|bibcode=2015OLEB...45..139M }}</ref> Under the same conditions, random ] chains are being formed, which are being continuously selected for their ability to integrate into the vesicle membrane. A further selection of the vesicles for their stability potentially leads to the development of functional peptide structures,<ref>{{Cite journal |last1=Dávila |first1=María J. |last2=Mayer |first2=Christian |date=2022 |title=Membrane Structure Obtained in an Experimental Evolution Process |journal=Life |language=en |volume=12 |issue=2 |pages=145 |doi=10.3390/life12020145 |doi-access=free |pmid=35207433 |pmc=8875328 |bibcode=2022Life...12..145D |issn=2075-1729}}</ref><ref>{{Cite journal |last=Mayer |first=Christian |date=2022 |title=Spontaneous Formation of Functional Structures in Messy Environments |journal=Life |language=en |volume=12 |issue=5 |pages=720 |doi=10.3390/life12050720 |doi-access=free |pmid=35629387 |pmc=9148140 |bibcode=2022Life...12..720M |issn=2075-1729}}</ref><ref>{{Cite journal |last1=Dávila |first1=María J. |last2=Mayer |first2=Christian |date=2023 |title=Structural Phenomena in a Vesicle Membrane Obtained through an Evolution Experiment: A Study Based on MD Simulations |journal=Life |language=en |volume=13 |issue=8 |pages=1735 |doi=10.3390/life13081735 |doi-access=free |pmid=37629592 |pmc=10455627 |bibcode=2023Life...13.1735D |issn=2075-1729}}</ref> associated with an increase in the survival rate of the vesicles. | |||
| * ] | |||
| * ] | |||
| == Producing biology == | |||
| * ] | |||
| * ] | |||
| === Energy and entropy === | |||
| * ] | |||
| * ] | |||
| {{further|Entropy}} | |||
| * ] | |||
| * ] | |||
| Life requires a loss of entropy, or disorder, as molecules organize themselves into living matter. At the same time, the emergence of life is associated with the formation of structures beyond a certain threshold of ].<ref>{{Cite journal |last=Mayer |first=Christian |date=2020-01-18 |title=Life in The Context of Order and Complexity |journal=Life |language=en |volume=10 |issue=1 |pages=5 |doi=10.3390/life10010005 |doi-access=free |issn=2075-1729 |pmc=7175320 |pmid=31963637|bibcode=2020Life...10....5M }}</ref> The emergence of life with increasing order and complexity does not contradict the second law of thermodynamics, which states that overall entropy never decreases, since a living organism creates order in some places (e.g. its living body) at the expense of an increase of entropy elsewhere (e.g. heat and waste production).<ref name="AP-2018">{{cite book |last1=Sharov |first1=Alexei A. |last2=Gordon |first2=Richard |title=Habitability of the Universe Before Earth: Life Before Earth |chapter=Life Before Earth |series=Astrobiology Exploring Life on Earth and Beyond |chapter-url=https://www.sciencedirect.com/science/article/pii/B9780128119402000113 |date=2018 |publisher=] |pages=265–296 |doi=10.1016/B978-0-12-811940-2.00011-3 |isbn=978-0-12-811940-2 |s2cid=117048600 |access-date=30 April 2022 |archive-date=30 April 2022 |archive-url=https://web.archive.org/web/20220430161329/https://www.sciencedirect.com/science/article/pii/B9780128119402000113 |url-status=live }}</ref><ref>{{cite journal |last1=Ladyman |first1=J. |last2=Lambert |first2=J. |last3=Weisner |first3=K. B. |title=What is a Complex System? |journal=European Journal of the Philosophy of Science |year=2013 |volume=3 |pages=33–67 |doi=10.1007/s13194-012-0056-8 |s2cid=18787276}}</ref><ref>{{cite journal |last1=Esposito |first1=M. |last2=Lindenberg |first2=Katja |author2-link=Katja Lindenberg |last3=Van den Broeck |first3=C. |year=2010 |title=Entropy production as correlation between system and reservoir |journal=] |volume=12 |issue=1 |page=013013 |doi=10.1088/1367-2630/12/1/013013 |arxiv=0908.1125 |bibcode=2010NJPh...12a3013E |s2cid=26657293}}</ref> | |||
| * ] | |||
| * ]s | |||
| Multiple sources of energy were available for chemical reactions on the early Earth. Heat from ] processes is a standard energy source for chemistry. Other examples include sunlight, lightning,<ref name="Follmann2009"/> atmospheric entries of micro-meteorites,<ref name="Bar-Nun Bar-Nun Bauer Sagan 1970">{{cite journal |last1=Bar-Nun |first1=A. |last2=Bar-Nun |first2=N. |last3=Bauer |first3=S. H. |last4=Sagan |first4=Carl |author4-link=Carl Sagan |title=Shock Synthesis of Amino Acids in Simulated Primitive Environments |journal=Science |volume=168 |issue=3930 |date=24 April 1970 |doi=10.1126/science.168.3930.470 |pages=470–473 |pmid=5436082 |bibcode=1970Sci...168..470B |s2cid=42467812}}</ref> and implosion of bubbles in sea and ocean waves.<ref name="Anbar 1968">{{cite journal |last=Anbar |first=Michael |title=Cavitation during Impact of Liquid Water on Water: Geochemical Implications |journal=] |volume=161 |issue=3848 |date=27 September 1968 |doi=10.1126/science.161.3848.1343 |pages=1343–1344 |pmid=17831346 |bibcode=1968Sci...161.1343A}}</ref> This has been confirmed by experiments<ref name="Dharmarathne Grieser 2016">{{cite journal |last1=Dharmarathne |first1=Leena |last2=Grieser |first2=Franz |title=Formation of Amino Acids on the Sonolysis of Aqueous Solutions Containing Acetic Acid, Methane, or Carbon Dioxide, in the Presence of Nitrogen Gas |journal=] |volume=120 |issue=2 |date=7 January 2016 |doi=10.1021/acs.jpca.5b11858 |pages=191–199 |pmid=26695890 |bibcode=2016JPCA..120..191D}}</ref><ref name="Patehebieke Zhao Wang Xu 2021">{{cite journal |last1=Patehebieke |first1=Yeersen |last2=Zhao |first2=Ze-Run |last3=Wang |first3=Su |last4=Xu |first4=Hao-Xing |last5=Chen |first5=Qian-Qian |last6=Wang |first6=Xiao |title=Cavitation as a plausible driving force for the prebiotic formation of N9 purine nucleosides |journal=Cell Reports Physical Science |volume=2 |issue=3 |year=2021 |doi=10.1016/j.xcrp.2021.100375 |page=100375 |bibcode=2021CRPS....200375P |s2cid=233662126|doi-access=free }}</ref> and simulations.<ref name="Kalson Furman Zeiri 2017">{{cite journal |last1=Kalson |first1=Natan-Haim |last2=Furman |first2=David |last3=Zeiri |first3=Yehuda |title=Cavitation-Induced Synthesis of Biogenic Molecules on Primordial Earth |journal=] |volume=3 |issue=9 |date=11 September 2017 |doi=10.1021/acscentsci.7b00325 |pages=1041–1049 |pmid=28979946 |pmc=5620973 |s2cid=21409351}}</ref> | |||
| * ] | |||
| Unfavorable reactions can be driven by highly favorable ones, as in the case of iron-sulfur chemistry. For example, this was probably important for ].{{efn|The reactions are: | |||
| * ] | |||
| :FeS + H<sub>2</sub>S → FeS<sub>2</sub> + 2H<sup>+</sup> + 2e<sup>−</sup> | |||
| * ] | |||
| :FeS + H<sub>2</sub>S + CO<sub>2</sub> → FeS<sub>2</sub> + HCOOH}} Carbon fixation by reaction of CO<sub>2</sub> with H<sub>2</sub>S via iron-sulfur chemistry is favorable, and occurs at neutral pH and 100 °C. Iron-sulfur surfaces, which are abundant near hydrothermal vents, can drive the production of small amounts of amino acids and other biomolecules.<ref name="Follmann2009"/> | |||
| * ] | |||
| * ] | |||
| === Chemiosmosis === | |||
| </div> | |||
| {{Further|Chemiosmosis}} | |||
| ] uses the chemiosmotic proton gradient to power ATP synthesis through ].]] | |||
| In 1961, ] proposed ] as a cell's primary system of energy conversion. The mechanism, now ubiquitous in living cells, powers energy conversion in micro-organisms and in the ] of eukaryotes, making it a likely candidate for early life.<ref>{{cite journal |last=Muller |first=Anthonie W. J. |year=1995 |title=Were the first organisms heat engines? A new model for biogenesis and the early evolution of biological energy conversion |journal=] |volume=63 |issue=2 |pages=193–231 |doi=10.1016/0079-6107(95)00004-7 |pmid=7542789 |doi-access=free}}</ref><ref>{{cite journal |last1=Muller |first1=Anthonie W. J. |first2=Dirk |last2=Schulze-Makuch |author-link2=Dirk Schulze-Makuch |year=2006 |title=Thermal energy and the origin of life |journal=] |volume=36 |issue=2 |pages=77–189 |bibcode=2006OLEB...36..177M |doi=10.1007/s11084-005-9003-4 |pmid=16642267 |s2cid=22179552}}</ref> Mitochondria produce ] (ATP), the energy currency of the cell used to drive cellular processes such as chemical syntheses. The mechanism of ATP synthesis involves a closed membrane in which the ] enzyme is embedded. The energy required to release strongly bound ATP has its origin in ]s that move across the membrane.<ref name="Junge Nelson 2015">{{cite journal |last1=Junge |first1=Wolfgang |last2=Nelson |first2=Nathan |title=ATP Synthase |journal=] |volume=84 |issue=1 |date=2 June 2015 |doi=10.1146/annurev-biochem-060614-034124 |pages=631–657 |pmid=25839341|doi-access=free }}</ref> In modern cells, those proton movements are caused by the pumping of ions across the membrane, maintaining an electrochemical gradient. In the first organisms, the gradient could have been provided by the difference in chemical composition between the flow from a hydrothermal vent and the surrounding seawater,<ref name="Lane 2015">{{cite book |last=Lane |first=Nick |author-link=Nick Lane |year=2015 |title=] |publisher=] |isbn=978-1-78125-036-5 |pages=129–140}}</ref> or perhaps meteoric quinones that were conducive to the development of chemiosmotic energy across lipid membranes if at a terrestrial origin.<ref name="Damer 2020">{{cite journal |last1=Damer |first1=Bruce |last2=Deamer |first2=David |date=2020-04-01 |title=The Hot Spring Hypothesis for an Origin of Life |journal=Astrobiology |volume=20 |issue=4 |pages=429–452 |doi=10.1089/ast.2019.2045 |pmc=7133448 |pmid=31841362 |bibcode=2020AsBio..20..429D}}</ref> | |||
| ] coupling in the membranes of a ]]] | |||
| {{clear}} | |||
| === PAH world hypothesis === | |||
| {{main|PAH world hypothesis}} | |||
| The PAH world hypothesis posits polycyclic aromatic hydrocarbons as precursors to the RNA world.<ref>{{cite journal |last1=d'Ischia |first1=Marco |last2=Manini |first2=Paola |last3=Moracci |first3=Marco |last4=Saladino |first4=Raffaele |last5=Ball |first5=Vincent |last6=Thissen |first6=Helmut |last7=Evans |first7=Richard A. |last8=Puzzarini |first8=Cristina |last9=Barone |first9=Vincenzo |display-authors=3 |date=21 August 2019 |title=Astrochemistry and Astrobiology: Materials Science in Wonderland? |journal=] |volume=20 |issue=17 |page=4079 |doi=10.3390/ijms20174079 |pmc=6747172 |pmid=31438518 |doi-access=free}}</ref> | |||
| {{excerpt|PAH world hypothesis|Paragraphs=1|files=0}} | |||
| === The RNA world === | |||
| {{Main|RNA world}} | |||
| The ] hypothesis describes an early Earth with self-replicating and catalytic RNA but no DNA or proteins.<ref>{{cite journal |last1=Benner |first1=S. A. |last2=Bell |first2=E. A. |last3=Biondi |first3=E. |last4=Brasser |first4=R. |last5=Carell |first5=T. |last6=Kim |first6=H.-J. |last7=Mojzsis |first7=S. J. |last8=Omran |first8=A. |last9=Pasek |first9=M. A. |last10=Trail |first10=D. |date=2020 |title=When Did Life Likely Emerge on Earth in an RNA-First Process? |journal=ChemSystemsChem |volume=2 |issue=2 |doi=10.1002/syst.201900035 |doi-access=free|arxiv=1908.11327 |bibcode=2020CSysC...2...35B }}</ref> Many researchers concur that an RNA world must have preceded the DNA-based life that now dominates.<ref name="RNA">* {{cite journal |last1=Copley |first1=Shelley D. |last2=Smith |first2=Eric |last3=Morowitz |first3=Harold J. |author-link3=Harold J. Morowitz |date=December 2007 |title=The origin of the RNA world: Co-evolution of genes and metabolism |url=http://tuvalu.santafe.edu/~desmith/PDF_pubs/Copley_BOG.pdf |url-status=live |journal=Bioorganic Chemistry |volume=35 |issue=6 |pages=430–443 |doi=10.1016/j.bioorg.2007.08.001 |pmid=17897696 |archive-url=https://web.archive.org/web/20130905070129/http://tuvalu.santafe.edu/~desmith/PDF_pubs/Copley_BOG.pdf |archive-date=5 September 2013 |access-date=8 June 2015 |quote=The proposal that life on Earth arose from an RNA world is widely accepted. |ref=none}} | |||
| * {{cite journal |last=Orgel |first=Leslie E. |author-link=Leslie Orgel |date=April 2003 |title=Some consequences of the RNA world hypothesis |journal=] |volume=33 |issue=2 |pages=211–218 |bibcode=2003OLEB...33..211O |doi=10.1023/A:1024616317965 |pmid=12967268 |quote=It now seems very likely that our familiar DNA/RNA/protein world was preceded by an RNA world... |s2cid=32779859 |ref=none}} | |||
| * {{harvnb|Robertson|Joyce|2012}}: "There is now strong evidence indicating that an RNA World did indeed exist before DNA- and protein-based life." | |||
| * {{harvnb|Neveu|Kim|Benner|2013}}: " has broad support within the community today."</ref> However, RNA-based life may not have been the first to exist.<ref name="Robertson2012">{{cite journal |last1=Robertson |first1=Michael P. |last2=Joyce |first2=Gerald F. |author-link2=Gerald Joyce |date=May 2012 |title=The origins of the RNA world |journal=] |volume=4 |issue=5 |page=a003608 |doi=10.1101/cshperspect.a003608 |pmc=3331698 |pmid=20739415}}</ref><ref name="Cech2012">{{cite journal |last=Cech |first=Thomas R. |author-link=Thomas Cech |date=July 2012 |title=The RNA Worlds in Context |journal=] |volume=4 |issue=7 |page=a006742 |doi=10.1101/cshperspect.a006742 |pmc=3385955 |pmid=21441585}}</ref> Another model echoes Darwin's "warm little pond" with cycles of wetting and drying.<ref>{{cite journal |last1=Pearce |first1=Ben K. D. |last2=Pudritz |first2=Ralph E. |last3=Semenov |first3=Dmitry A. |last4=Henning |first4=Thomas K. |date=24 October 2017 |title=Origin of the RNA world: The fate of nucleobases in warm little ponds |journal=] |volume=114 |issue=43 |pages=11327–11332 |doi=10.1073/pnas.1710339114 |pmid=28973920 |pmc=5664528 |arxiv=1710.00434 |bibcode=2017PNAS..11411327P |doi-access=free}}</ref> | |||
| RNA is central to the translation process. Small RNAs can catalyze all the chemical groups and information transfers required for life.<ref name="Cech2012"/><ref name="Yarus2011">{{cite journal |last=Yarus |first=Michael |date=April 2011 |title=Getting Past the RNA World: The Initial Darwinian Ancestor |journal=Cold Spring Harbor Perspectives in Biology |volume=3 |issue=4 |page=a003590 |doi=10.1101/cshperspect.a003590 |pmc=3062219 |pmid=20719875}}</ref> RNA both expresses and maintains genetic information in modern organisms; and the chemical components of RNA are easily synthesized under the conditions that approximated the early Earth, which were very different from those that prevail today. The structure of the ] has been called the "smoking gun", with a central core of RNA and no amino acid side chains within 18 ] of the ] that catalyzes peptide bond formation.<ref>{{harvnb|Voet|Voet|2004|p=29}}</ref><ref name="Robertson2012"/><ref>{{cite journal |last1=Fox |first1=George.E. |date=9 June 2010 |title=Origin and evolution of the ribosome |journal=] |volume=2 |issue=9(a003483) |page=a003483 |doi=10.1101/cshperspect.a003483 |pmc=2926754 |pmid=20534711 |doi-access=free}}</ref> | |||
| The concept of the RNA world was proposed in 1962 by ],<ref>{{cite journal |last1=Neveu |first1=Marc |last2=Kim |first2=Hyo-Joong |last3=Benner |first3=Steven A. |date=22 April 2013 |title=The 'Strong' RNA World Hypothesis: Fifty Years Old |journal=] |volume=13 |issue=4 |pages=391–403 |bibcode=2013AsBio..13..391N |doi=10.1089/ast.2012.0868 |pmid=23551238}}</ref> and the term was coined by ] in 1986.<ref name="Cech2012"/><ref>{{cite journal |last=Gilbert |first=Walter |author-link=Walter Gilbert |date=20 February 1986 |title=Origin of life: The RNA world |journal=Nature |volume=319 |issue=6055 |page=618 |bibcode=1986Natur.319..618G |doi=10.1038/319618a0 |doi-access=free |s2cid=8026658}}</ref> There were initial difficulties in the explanation of the abiotic synthesis of the nucleotides cytosine and uracil.<ref>{{cite journal |last=Orgel |first=Leslie E. |date=October 1994 |title=The origin of life on Earth |journal=Scientific American |volume=271 |issue=4 |pages=76–83 |bibcode=1994SciAm.271d..76O |doi=10.1038/scientificamerican1094-76 |pmid=7524147}}</ref> Subsequent research has shown possible routes of synthesis; for example, formamide produces all four ]s and other biological molecules when warmed in the presence of various terrestrial minerals.<ref name="Saladino2012"/><ref name="Saladino2012b"/> | |||
| ]s, and in turn to an ].]] | |||
| ] can function as both code and catalyst for further RNA replication, i.e. it can be autocatalytic. ] has shown that certain catalytic RNAs can join smaller RNA sequences together, creating the potential for self-replication. The RNA replication systems, which include two ribozymes that catalyze each other's synthesis, showed a doubling time of the product of about one hour, and were subject to natural selection under the experimental conditions.<ref>{{cite journal |last1=Lincoln |first1=Tracey A. |last2=Joyce |first2=Gerald F. |date=27 February 2009 |title=Self-Sustained Replication of an RNA Enzyme |journal=] |volume=323 |issue=5918 |pages=1229–1232 |bibcode=2009Sci...323.1229L |doi=10.1126/science.1167856 |pmc=2652413 |pmid=19131595}}</ref><ref name="Joyce2009">{{cite journal |last=Joyce |first=Gerald F. |year=2009 |title=Evolution in an RNA world |journal=Cold Spring Harbor Perspectives in Biology |volume=74 |issue=Evolution: The Molecular Landscape |pages=17–23 |doi=10.1101/sqb.2009.74.004 |pmc=2891321 |pmid=19667013}}</ref><ref name="Robertson2012"/> If such conditions were present on early Earth, then natural selection would favor the proliferation of such ]s, to which further functionalities could be added.<ref>{{cite web |last=Szostak |first=Jack W. |author-link=Jack W. Szostak |date=5 February 2015 |title=The Origins of Function in Biological Nucleic Acids, Proteins, and Membranes |url=http://www.hhmi.org/research/origins-cellular-life |url-status=live |archive-url=https://web.archive.org/web/20150714092225/http://www.hhmi.org/research/origins-cellular-life |archive-date=14 July 2015 |access-date=16 June 2015 |publisher=] |location=Chevy Chase, Maryland}}</ref><ref name="Bernstein">{{cite journal |last1=Bernstein |first1=Harris |last2=Byerly |first2=Henry C. |last3=Hopf |first3=Frederick A. |last4=Michod |first4=Richard A. |last5=Vemulapalli |first5=G. Krishna |display-authors=3 |date=June 1983 |title=The Darwinian Dynamic |journal=] |volume=58 |issue=2 |pages=185–207 |doi=10.1086/413216 |jstor=2828805 |s2cid=83956410}}</ref><ref name="Michod 1999">{{harvnb|Michod|1999}}</ref> Self-assembly of RNA may occur spontaneously in hydrothermal vents.<ref>{{cite arXiv |eprint=1305.5581v1 |class=q-bio.BM |first=Stan |last=Palasek |title=Primordial RNA Replication and Applications in PCR Technology |date=23 May 2013}}</ref><ref name="pmid16044244">{{cite journal |last1=Vlassov |first1=Alexander V. |last2=Kazakov |first2=Sergei A. |last3=Johnston |first3=Brian H. |last4=Landweber |first4=Laura F. |display-authors=3 |date=August 2005 |title=The RNA World on Ice: A New Scenario for the Emergence of RNA Information |journal=] |volume=61 |issue=2 |pages=264–273 |bibcode=2005JMolE..61..264V |doi=10.1007/s00239-004-0362-7 |pmid=16044244 |s2cid=21096886}}</ref><ref>{{cite journal |last1=Nussinov |first1=Mark D. |last2=Otroshchenko |first2=Vladimir A. |last3=Santoli |first3=Salvatore |year=1997 |title=The emergence of the non-cellular phase of life on the fine-grained clayish particles of the early Earth's regolith |journal=] |volume=42 |issue=2–3 |pages=111–118 |doi=10.1016/S0303-2647(96)01699-1 |pmid=9184757|bibcode=1997BiSys..42..111N }}</ref> A preliminary form of tRNA could have assembled into such a replicator molecule.<ref name="EL-20210302">{{cite journal |last1=Kühnlein |first1=Alexandra |last2=Lanzmich |first2=Simon A. |last3=Brun |first3=Dieter |date=2 March 2021 |title=tRNA sequences can assemble into a replicator |journal=] |volume=10 |doi=10.7554/eLife.63431 |pmc=7924937 |pmid=33648631 |doi-access=free}}</ref> | |||
| Possible precursors to protein synthesis include the synthesis of short peptide cofactors or the self-catalysing duplication of RNA. It is likely that the ancestral ribosome was composed entirely of RNA, although some roles have since been taken over by proteins. Major remaining questions on this topic include identifying the selective force for the evolution of the ribosome and determining how the genetic code arose.<ref name="Noller2012">{{cite journal |last=Noller |first=Harry F. |author-link=Harry F. Noller |date=April 2012 |title=Evolution of protein synthesis from an RNA world. |journal=] |volume=4 |issue=4 |page=a003681 |doi=10.1101/cshperspect.a003681 |pmc=3312679 |pmid=20610545|bibcode=2012CSHPB...4.3681N }}</ref> | |||
| ] has argued that "no compelling scenarios currently exist for the origin of replication and translation, the key processes that together comprise the core of biological systems and the apparent pre-requisite of biological evolution. The RNA World concept might offer the best chance for the resolution of this conundrum but so far cannot adequately account for the emergence of an efficient RNA replicase or the translation system."<ref name="pmc1892545">{{cite journal |last=Koonin |first=Eugene V. |author-link=Eugene Koonin |date=31 May 2007 |title=The cosmological model of eternal inflation and the transition from chance to biological evolution in the history of life |journal=] |volume=2 |page=15 |doi=10.1186/1745-6150-2-15 |pmc=1892545 |pmid=17540027 |doi-access=free }}</ref> | |||
| === From RNA to directed protein synthesis === | |||
| In line with the RNA world hypothesis, much of modern biology's templated protein biosynthesis is done by RNA molecules—namely tRNAs and the ribosome (consisting of both protein and rRNA components). The most central reaction of peptide bond synthesis is understood to be carried out by base catalysis by the 23S rRNA domain V.<ref name=":1">{{Cite journal |last1=Tamura |first1=K. |last2=Alexander |first2=R. W. |date=2004-05-01 |title=Peptide synthesis through evolution |journal=Cellular and Molecular Life Sciences |language=en |volume=61 |issue=11 |pages=1317–1330 |doi=10.1007/s00018-004-3449-9 |pmid=15170510 |issn=1420-9071|pmc=11138682 }}</ref> Experimental evidence has demonstrated successful di- and tripeptide synthesis with a system consisting of only aminoacyl phosphate adaptors and RNA guides, which could be a possible stepping stone between an RNA world and modern protein synthesis.<ref name=":1" /><ref>{{Cite journal |last1=Tamura |first1=Koji |last2=Schimmel |first2=Paul |date=2003-07-22 |title=Peptide synthesis with a template-like RNA guide and aminoacyl phosphate adaptors |journal=Proceedings of the National Academy of Sciences |language=en |volume=100 |issue=15 |pages=8666–8669 |doi=10.1073/pnas.1432909100 |doi-access=free |issn=0027-8424 |pmc=166369 |pmid=12857953|bibcode=2003PNAS..100.8666T }}</ref> Aminoacylation ribozymes that can charge tRNAs with their cognate amino acids have also been selected in in vitro experimentation.<ref name=":2">{{Cite journal |last1=Pressman |first1=Abe D. |last2=Liu |first2=Ziwei |last3=Janzen |first3=Evan |last4=Blanco |first4=Celia |last5=Müller |first5=Ulrich F. |last6=Joyce |first6=Gerald F. |last7=Pascal |first7=Robert |last8=Chen |first8=Irene A. |date=2019-04-17 |title=Mapping a Systematic Ribozyme Fitness Landscape Reveals a Frustrated Evolutionary Network for Self-Aminoacylating RNA |journal=Journal of the American Chemical Society |language=en |volume=141 |issue=15 |pages=6213–6223 |doi=10.1021/jacs.8b13298 |issn=0002-7863 |pmc=6548421 |pmid=30912655|bibcode=2019JAChS.141.6213P }}</ref> The authors also extensively mapped fitness landscapes within their selection to find that chance emergence of active sequences was more important that sequence optimization.<ref name=":2" /> | |||
| === Early functional peptides === | |||
| The first proteins would have had to arise without a fully-fledged system of protein biosynthesis. As discussed above, numerous mechanisms for the prebiotic synthesis of polypeptides exist. However, these random sequence peptides would not have likely had biological function. Thus, significant study has gone into exploring how early functional proteins could have arisen from random sequences. First, some evidence on hydrolysis rates shows that abiotically plausible peptides likely contained significant "nearest-neighbor" biases.<ref>{{Cite journal |last1=Tyagi |first1=Sanjay |last2=Ponnamperuma |first2=Cyril |date=1990-05-01 |title=Nonrandomness in prebiotic peptide synthesis |journal=Journal of Molecular Evolution |language=en |volume=30 |issue=5 |pages=391–399 |doi=10.1007/BF02101111 |pmid=2111852 |bibcode=1990JMolE..30..391T |issn=1432-1432}}</ref> This could have had some effect on early protein sequence diversity. In other work by Anthony Keefe and Jack Szostak, ] selection on a library of 6*10<sup>12</sup> 80-mers was used to search for sequences with ATP binding activity. They concluded that approximately 1 in 10<sup>11</sup> random sequences had ATP binding function.<ref>{{Cite journal |title=Functional proteins from a random-sequence library |doi=10.1038/35070613 |pmc=4476321 |pmid=11287961 | date=2001 | last1=Keefe | first1=Anthony D. | last2=Szostak | first2=Jack W. | journal=Nature | volume=410 | issue=6829 | pages=715–718 |bibcode=2001Natur.410..715K }}</ref> While this is a single example of functional frequency in the random sequence space, the methodology can serve as a powerful simulation tool for understanding early protein evolution.<ref>{{Cite journal |last1=Tong |first1=Cher Ling |last2=Lee |first2=Kun-Hwa |last3=Seelig |first3=Burckhard |date=June 2021 |title=De novo proteins from random sequences through in vitro evolution |journal=Current Opinion in Structural Biology |language=en |volume=68 |pages=129–134 |doi=10.1016/j.sbi.2020.12.014 |pmc=8222087 |pmid=33517151|bibcode=2021COStB..68..129T }}</ref> | |||
| === Phylogeny and LUCA === | |||
| {{further|Last universal common ancestor}} | |||
| Starting with the work of ] from 1977, ] studies have placed the last universal common ancestor (LUCA) of all modern life-forms between Bacteria and a clade formed by Archaea and ] in the phylogenetic tree of life. It lived over 4 Gya.<ref>{{cite book |url=https://www.springer.com/life+sciences/microbiology/book/978-0-387-98771-2 |title=The ''Archaea'' and the Deeply Branching and Phototrophic ''Bacteria'' |publisher=] |year=2001 |isbn=978-0-387-21609-6 |editor1-last=Boone |editor1-first=David R. |series=Bergey's Manual of Systematic Bacteriology |editor2-last=Castenholz |editor2-first=Richard W. |editor3-last=Garrity |editor3-first=George M. |archive-url=https://web.archive.org/web/20141225112809/http://www.springer.com/life+sciences/microbiology/book/978-0-387-98771-2 |archive-date=25 December 2014 |url-status=live}}{{page needed |date=June 2014}}</ref><ref name="Woese Fox 1977">{{cite journal |last1=Woese |first1=C. R. |last2=Fox |first2=G. E. |year=1977 |title=Phylogenetic structure of the prokaryotic domain: the primary kingdoms. |journal=] |volume=7 |issue=11 |pages=5088–5090 |bibcode=1977PNAS...74.5088W |doi=10.1073/pnas.74.11.5088 |pmc=432104 |pmid=270744 |doi-access=free}}</ref> A minority of studies have placed the LUCA in Bacteria, proposing that Archaea and Eukaryota are evolutionarily derived from within Eubacteria;<ref>{{cite journal |last1=Valas |first1=R. E. |last2=Bourne |first2=P. E. |year=2011 |title=The origin of a derived superkingdom: how a gram-positive bacterium crossed the desert to become an archaeon |journal=] |volume=6 |page=16 |doi=10.1186/1745-6150-6-16 |pmc=3056875 |pmid=21356104 |doi-access=free }}</ref> ] suggested in 2006 that the phenotypically diverse bacterial phylum ] contained the LUCA.<ref name="CS2">{{cite journal |last=Cavalier-Smith |first=Thomas |author-link=Thomas Cavalier-Smith |year=2006 |title=Rooting the tree of life by transition analyses |journal=] |volume=1 |page=19 |doi=10.1186/1745-6150-1-19 |pmc=1586193 |pmid=16834776 |doi-access=free }}</ref> | |||
| <gallery mode="packed" heights="200px"> | |||
| File:Phylogenetic tree of life LUCA.svg|] showing the ] (LUCA) at the root. The major clades are the ] on one hand, and the ] and ] on the other. | |||
| </gallery> | |||
| In 2016, a set of 355 genes likely present in the LUCA was identified. A total of 6.1 million prokaryotic genes from Bacteria and Archaea were sequenced, identifying 355 protein clusters from among 286,514 protein clusters that were probably common to the LUCA. The results suggest that the LUCA was ] with a Wood–Ljungdahl (reductive Acetyl-CoA) pathway, nitrogen- and carbon-fixing, thermophilic. Its ] suggest dependence upon an environment rich in hydrogen, carbon dioxide, iron, and ]s. Its genetic material was probably DNA, requiring the 4-nucleotide genetic code, messenger RNA, transfer RNA, and ribosomes to translate the code into proteins such as enzymes. LUCA likely inhabited an anaerobic hydrothermal vent setting in a geochemically active environment. It was evidently already a complex organism, and must have had precursors; it was not the first living thing.<ref name="Weiss Sousa Mrnjavac 2016">{{cite journal |last1=Weiss |first1=M. C. |last2=Sousa |first2=F. L. |last3=Mrnjavac |first3=N. |last4=Neukirchen |first4=S. |last5=Roettger |first5=M. |last6=Nelson-Sathi |first6=S. |last7=Martin |first7=W.F. |year=2016 |title=The physiology and habitat of the last universal common ancestor |url=https://www.almendron.com/tribuna/wp-content/uploads/2019/10/the-physiology-and-habitat-of-the-last-universal-common-ancestor.pdf |journal=] |volume=1 |issue=9 |page=16116 |doi=10.1038/NMICROBIOL.2016.116 |pmid=27562259 |s2cid=2997255 |access-date=21 September 2022 |archive-date=29 January 2023 |archive-url=https://web.archive.org/web/20230129185028/https://www.almendron.com/tribuna/wp-content/uploads/2019/10/the-physiology-and-habitat-of-the-last-universal-common-ancestor.pdf |url-status=live }}</ref><ref name="Nature 2016">{{cite journal |title=Early life liked it hot |journal=Nature |volume=535 |issue=7613 |year=2016 |doi=10.1038/535468b |page=468 |s2cid=49905802|doi-access=free }}</ref> The physiology of LUCA has been in dispute.<ref>{{cite journal |last1=Gogarten |first1=Johann Peter |last2=Deamer |first2=David |author2-link=David W. Deamer |date=2016-11-25 |title=Is LUCA a thermophilic progenote? |url=https://zenodo.org/record/895471 |journal=Nature Microbiology |volume=1 |issue=12 |page=16229 |doi=10.1038/nmicrobiol.2016.229 |pmid=27886195 |s2cid=205428194 |access-date=21 September 2022 |archive-date=3 April 2020 |archive-url=https://web.archive.org/web/20200403040656/https://zenodo.org/record/895471 |url-status=live }}</ref><ref>{{cite journal |last1=Catchpole |first1=Ryan |last2=Forterre |first2=Patrick |date=2019 |title=The evolution of Reverse Gyrase suggests a non-hyperthermophilic Last Universal Common Ancestor |url=https://academic.oup.com/mbe/article/36/12/2737/5545984 |journal=Molecular Biology and Evolution |volume=36 |issue=12 |pages=2737–2747 |doi=10.1093/molbev/msz180 |pmid=31504731 |pmc=6878951 |access-date=18 September 2022 |archive-date=27 January 2023 |archive-url=https://web.archive.org/web/20230127162358/https://academic.oup.com/mbe/article/36/12/2737/5545984 |url-status=live }}</ref><ref>{{cite journal |last1=Berkemer |first1=Sarah J. |last2=McGlynn |first2=Shawn E |date=August 8, 2020 |title=A New Analysis of Archaea–Bacteria Domain Separation: Variable Phylogenetic Distance and the Tempo of Early Evolution |url=https://academic.oup.com/mbe/article/37/8/2332/5818498 |journal=Molecular Biology and Evolution |volume=37 |issue=8 |pages=2332–2340 |doi=10.1093/molbev/msaa089 |pmc=7403611 |pmid=32316034 |access-date=21 September 2022 |archive-date=27 January 2023 |archive-url=https://web.archive.org/web/20230127163913/https://academic.oup.com/mbe/article/37/8/2332/5818498 |url-status=live }}</ref> Previous research identified 60 proteins common to all life.<ref>{{cite journal | url=https://pubmed.ncbi.nlm.nih.gov/15035042/ | pmid=15035042 | date=2003 | last1=Koonin | first1=E. V. | title=Comparative genomics, minimal gene-sets and the last universal common ancestor | journal=Nature Reviews. Microbiology | volume=1 | issue=2 | pages=127–136 | doi=10.1038/nrmicro751 }}</ref> | |||
| <gallery mode="packed" heights="300px"> | |||
| File:LUCA systems and environment.svg|LUCA systems and environment included the ].<ref name="Weiss Sousa Mrnjavac 2016"/> | |||
| </gallery> | |||
| Leslie Orgel argued that early translation machinery for the genetic code would be susceptible to ]. Geoffrey Hoffmann however showed that such machinery can be stable in function against "Orgel's paradox".<ref>{{cite journal |last=Hoffmann |first=Geoffrey W. |author-link=Geoffrey W. Hoffmann |date=25 June 1974 |title=On the origin of the genetic code and the stability of the translation apparatus |journal=] |volume=86 |issue=2 |pages=349–362 |doi=10.1016/0022-2836(74)90024-2 |pmid=4414916}}</ref><ref>{{cite journal |last=Orgel |first=Leslie E. |date=April 1963 |title=The Maintenance of the Accuracy of Protein Synthesis and its Relevance to Ageing |journal=] |volume=49 |issue=4 |pages=517–521 |bibcode=1963PNAS...49..517O |doi=10.1073/pnas.49.4.517 |pmc=299893 |pmid=13940312 |doi-access=free}}</ref><ref>{{cite journal |last=Hoffmann |first=Geoffrey W. |date=October 1975 |title=The Stochastic Theory of the Origin of the Genetic Code |journal=] |volume=26 |pages=123–144 |bibcode=1975ARPC...26..123H |doi=10.1146/annurev.pc.26.100175.001011}}</ref> Metabolic reactions that have also been inferred in LUCA are the incomplete ], ], the ], ], ], and ].<ref>{{Cite journal |last1=Harrison |first1=Stuart A. |last2=Palmeira |first2=Raquel Nunes |last3=Halpern |first3=Aaron |last4=Lane |first4=Nick |date=2022-11-01 |title=A biophysical basis for the emergence of the genetic code in protocells |journal=Biochimica et Biophysica Acta (BBA) - Bioenergetics |volume=1863 |issue=8 |page=148597 |doi=10.1016/j.bbabio.2022.148597 |pmid=35868450 |s2cid=250707510 |doi-access=free }}</ref><ref>{{Cite journal |last1=Harrison |first1=Stuart A. |last2=Lane |first2=Nick |date=2018-12-12 |title=Life as a guide to prebiotic nucleotide synthesis |journal=Nature Communications |volume=9 |issue=1 |page=5176 |doi=10.1038/s41467-018-07220-y |pmid=30538225 |pmc=6289992 |bibcode=2018NatCo...9.5176H}}</ref> | |||
| == Suitable geological environments == | |||
| {{further|Alternative abiogenesis scenarios}} | |||
| A variety of ] for an origin of life. These theories are often in competition with one another as there are many differing views of prebiotic compound availability, geophysical setting, and early life characteristics. The first organism on Earth likely looked different from ]. Between the first appearance of life and where all modern phylogenies began branching, an unknown amount of time passed, with unknown gene transfers, extinctions, and evolutionary adaptation to various environmental niches.<ref>{{Cite journal |last1=Cantine |first1=Marjorie D. |last2=Fournier |first2=Gregory P. |date=2018-03-01 |title=Environmental Adaptation from the Origin of Life to the Last Universal Common Ancestor |journal=Origins of Life and Evolution of Biospheres |volume=48 |issue=1 |pages=35–54 |doi=10.1007/s11084-017-9542-5 |pmid=28685374 |bibcode=2018OLEB...48...35C |hdl=1721.1/114219 |s2cid=254888920 |url=https://link.springer.com/article/10.1007/s11084-017-9542-5 |hdl-access=free |archive-date=31 January 2024 |archive-url=https://web.archive.org/web/20240131154929/https://link.springer.com/article/10.1007/s11084-017-9542-5 |url-status=live }}</ref> One major shift is believed to be from the RNA world to an RNA-DNA-protein world. Modern phylogenies provide more pertinent genetic evidence about LUCA than about its precursors.<ref>{{Cite journal |last=Mat |first=Wai-Kin |date=May 1, 2008 |title=The genomics of LUCA |url=https://article.imrpress.com/bri/Landmark/articles/pdf/Landmark3103.pdf |journal=Frontiers in Bioscience |volume=13 |issue=14 |pages=5605–5613 |doi=10.2741/3103 |pmid=18508609 |access-date=8 December 2023 |archive-date=16 June 2022 |archive-url=https://web.archive.org/web/20220616034359/https://article.imrpress.com/bri/Landmark/articles/pdf/Landmark3103.pdf |url-status=live }}</ref> | |||
| The most popular hypotheses for settings for the origin of life are deep sea hydrothermal vents and surface bodies of water. Surface waters can be classified into hot springs, moderate temperature lakes and ponds, and cold settings. | |||
| === Deep sea hydrothermal vents === | |||
| ==== Hot fluids ==== | |||
| {{further|Hydrothermal vent}} | |||
| ] are putative fossilized ]s, found in white smoker ] precipitates. They may have lived as early as 4.28 Gya (billion years ago), relatively soon after the ] 4.41 Gya, not long after the ] 4.54 Gya.<ref name="NAT-20170301"/>]] | |||
| Early micro-fossils may have come from a hot world of gases such as methane, ammonia, carbon dioxide, and ], toxic to much current life.<ref>{{cite book |last=Brasier |first=M. D. |year=2012 |title=Secret Chambers: The Inside Story of Cells and Complex Life |publisher=Oxford University Press |page=298}}</ref> Analysis of the ] places thermophilic and hyperthermophilic bacteria and archaea closest to the root, suggesting that life may have evolved in a hot environment.<ref>Ward, Peter & ], op cit, p. 42</ref> The deep sea or alkaline hydrothermal vent theory posits that life began at submarine hydrothermal vents.<ref name="Colín-García 2016">{{cite journal |last1=Colín-García |first1=M. |last2=Heredia |first2=A. |last3=Cordero |first3=G. |last4=Camprubí |first4=A. |last5=Negrón-Mendoza |first5=A. |last6=Ortega-Gutiérrez |first6=F. |last7=Berald |first7=H. |last8=Ramos-Bernal |first8=S. |display-authors=3 |year=2016 |title=Hydrothermal vents and prebiotic chemistry: a review |url=http://boletinsgm.igeolcu.unam.mx/bsgm/index.php/component/content/article/309-sitio/articulos/cuarta-epoca/6803/1620-6803-13-colin |journal=Boletín de la Sociedad Geológica Mexicana |volume=68 |issue=3 |pages=599–620 |url-status=live |archive-url=https://web.archive.org/web/20170818175803/http://boletinsgm.igeolcu.unam.mx/bsgm/index.php/component/content/article/309-sitio/articulos/cuarta-epoca/6803/1620-6803-13-colin |archive-date=18 August 2017 |doi=10.18268/BSGM2016v68n3a13 |doi-access=free}}</ref><ref name="hydrothermal vents NASA 2014">{{cite web |url=https://astrobiology.nasa.gov/articles/2014/6/24/hydrothermal-vents-could-explain-chemical-precursors-to-life/ |title=Hydrothermal Vents Could Explain Chemical Precursors to Life |last=Schirber |first=Michael |date=24 June 2014 |website=NASA Astrobiology: Life in the Universe |publisher=NASA |access-date=19 June 2015 |archive-url=https://web.archive.org/web/20141129051724/http://astrobiology.nasa.gov/articles/2014/6/24/hydrothermal-vents-could-explain-chemical-precursors-to-life/ |archive-date=29 November 2014}}</ref> ] and ] have suggested "that life evolved in structured iron monosulphide precipitates in a seepage site hydrothermal mound at a redox, pH, and temperature gradient between sulphide-rich hydrothermal fluid and iron(II)-containing waters of the Hadean ocean floor. The naturally arising, three-dimensional compartmentation observed within fossilized seepage-site metal sulphide precipitates indicates that these inorganic compartments were the precursors of cell walls and membranes found in free-living prokaryotes. The known capability of FeS and NiS to catalyze the synthesis of the acetyl-methylsulphide from carbon monoxide and methylsulphide, constituents of hydrothermal fluid, indicates that pre-biotic syntheses occurred at the inner surfaces of these metal-sulphide-walled compartments".<ref name="Martin2003">{{cite journal |last1=Martin |first1=William |author-link1=William F. Martin |last2=Russell |first2=Michael J. |date=29 January 2003 |title=On the origins of cells: a hypothesis for the evolutionary transitions from abiotic geochemistry to chemoautotrophic prokaryotes, and from prokaryotes to nucleated cells |journal=] |volume=358 |issue=1429 |pages=59–83; discussion 83–85 |doi=10.1098/rstb.2002.1183 |pmid=12594918 |pmc=1693102}}</ref> | |||
| These form where hydrogen-rich fluids emerge from below the sea floor, as a result of ] of ultra-] ] with seawater and a pH interface with carbon dioxide-rich ocean water. The vents form a sustained chemical energy source derived from redox reactions, in which electron donors (molecular hydrogen) react with electron acceptors (carbon dioxide); see ]. These are ]s.<ref name="Colín-García 2016"/>{{efn|The reactions are:<br/> | |||
| '''Reaction 1''': Fayalite + water → magnetite + aqueous silica + hydrogen | |||
| :3Fe<sub>2</sub>SiO<sub>4</sub> + 2H<sub>2</sub>O → 2Fe<sub>3</sub>O<sub>4</sub> + 3SiO<sub>2</sub> + 2H<sub>2</sub> | |||
| '''Reaction 2''': Forsterite + aqueous silica → serpentine | |||
| :3Mg<sub>2</sub>SiO<sub>4</sub> + SiO<sub>2</sub> + 4H<sub>2</sub>O → 2Mg<sub>3</sub>Si<sub>2</sub>O<sub>5</sub>(OH)<sub>4</sub> | |||
| '''Reaction 3''': Forsterite + water → serpentine + brucite | |||
| :2Mg<sub>2</sub>SiO<sub>4</sub> + 3H<sub>2</sub>O → Mg<sub>3</sub>Si<sub>2</sub>O<sub>5</sub>(OH)<sub>4</sub> + Mg(OH)<sub>2</sub> | |||
| Reaction 3 describes the hydration of olivine with water only to yield ] and Mg(OH)<sub>2</sub> (]). Serpentine is stable at high pH in the presence of brucite like calcium silicate hydrate, (]) phases formed along with ] (Ca(OH)<sub>2</sub>) in hardened ] paste after the hydration of ] (Ca<sub>2</sub>SiO<sub>4</sub>), the artificial calcium equivalent of forsterite. | |||
| Analogy of reaction 3 with belite hydration in ordinary Portland cement: ''Belite + water → C-S-H phase + portlandite'' | |||
| :2 Ca<sub>2</sub>SiO<sub>4</sub> + 4 H<sub>2</sub>O → 3 CaO · 2 SiO<sub>2</sub> · 3 H<sub>2</sub>O + Ca(OH)<sub>2</sub>}} | |||
| ==== Chemiosmotic gradient ==== | |||
| {{further|Hydrothermal vent|Chemiosmosis#Emergence of chemiosmosis}} | |||
| ] | |||
| Russell demonstrated that alkaline vents created an abiogenic ] chemiosmotic gradient,<ref name="Martin2003"/> ideal for abiogenesis. Their microscopic compartments "provide a natural means of concentrating organic molecules," composed of iron-sulfur minerals such as ], endowed these mineral cells with the catalytic properties envisaged by ].<ref name="Lane 2009">{{harvnb|Lane|2009}}</ref> This movement of ions across the membrane depends on a combination of two factors: | |||
| # ] force caused by concentration gradient—all particles including ions tend to diffuse from higher concentration to lower. | |||
| # Electrostatic force caused by electrical potential gradient—]s like ]s H<sup>+</sup> tend to diffuse down the electrical potential, ]s in the opposite direction. | |||
| These two gradients taken together can be expressed as an electrochemical gradient, providing energy for abiogenic synthesis. The proton motive force can be described as the measure of the potential energy stored as a combination of proton and voltage gradients across a membrane (differences in proton concentration and electrical potential).<ref name="Lane 2015"/> | |||
| <!--File:Champagne vent white smokers.jpg is at the top of this article already--> | |||
| The surfaces of mineral particles inside deep-ocean hydrothermal vents have catalytic properties similar to those of enzymes and can create simple organic molecules, such as ] (CH<sub>3</sub>OH) and ], ], and ]s out of the dissolved CO<sub>2</sub> in the water, if driven by an applied voltage or by reaction with H<sub>2</sub> or H<sub>2</sub>S.<ref name="organics">{{cite press release |last=Usher |first=Oli |date=27 April 2015 |title=Chemistry of seabed's hot vents could explain emergence of life |url=https://www.ucl.ac.uk/silva/mathematical-physical-sciences/maps-news-publication/maps1526 |publisher=] |access-date=19 June 2015 |archive-url=https://web.archive.org/web/20150620012231/https://www.ucl.ac.uk/silva/mathematical-physical-sciences/maps-news-publication/maps1526 |archive-date=20 June 2015 }}</ref><ref>{{cite journal |last1=Roldan |first1=Alberto |last2=Hollingsworth |first2=Nathan |last3=Roffey |first3=Anna |last4=Islam |first4=Husn-Ubayda |last5=Goodall |first5=Josephine B. M. |last6=Catlow |first6=C. Richard A. |author6-link=Richard Catlow |last7=Darr |first7=Jawwad A. |last8=Bras |first8=Wim |last9=Sankar |first9=Gopinathan |last10=Holt |first10=Katherine B. |last11=Hogarth |first11=Graeme |last12=de Leeuw |first12=Nora Henriette |display-authors=3 |date=May 2015 |title=Bio-inspired CO2 conversion by iron sulfide catalysts under sustainable conditions |url=http://pubs.rsc.org/en/content/articlepdf/2015/cc/c5cc02078f |journal=] |volume=51 |issue=35 |pages=7501–7504 |doi=10.1039/C5CC02078F |pmid=25835242 |access-date=2015-06-19 |url-status=live |archive-url=https://web.archive.org/web/20150620003943/http://pubs.rsc.org/en/content/articlepdf/2015/cc/c5cc02078f |archive-date=20 June 2015 |doi-access=free}}</ref> | |||
| Starting in 1985, researchers proposed that life arose at hydrothermal vents,<ref>{{cite journal |last1=Baross |first1=J. A. |last2=Hoffman |first2=S. E. |year= 1985 |title=Submarine hydrothermal vents and associated gradient environments as sites for the origin and evolution of life |journal=] |volume=15 |issue=4 |pages=327–345 |doi=10.1007/bf01808177 |bibcode=1985OrLi...15..327B |s2cid=4613918}}</ref><ref>{{cite journal |last1=Russell |first1=M. J. |last2= Hall |first2=A. J. |year=1997 |title=The emergence of life from iron monosulphide bubbles at a submarine hydrothermal redox and pH front |journal=] |volume=154 |issue=3 |pages=377–402 |doi=10.1144/gsjgs.154.3.0377 |pmid=11541234 |bibcode=1997JGSoc.154..377R |s2cid=24792282}}</ref> that spontaneous chemistry in the Earth's crust driven by rock–water interactions at disequilibrium thermodynamically underpinned life's origin<ref>{{cite journal |last1=Amend |first1=J. P. |last2= LaRowe |first2=D. E. |last3=McCollom |first3=T. M. |last4=Shock |first4=E. L. |year=2013 |title=The energetics of organic synthesis inside and outside the cell |journal= ] |volume=368 |issue=1622 |page=20120255 |doi=10.1098/rstb.2012.0255 |pmid=23754809 |pmc=3685458}}</ref><ref>{{cite journal |last1=Shock |first1=E. L. |last2=Boyd |first2=E. S. |year=2015 |title=Geomicrobiology and microbial geochemistry:principles of geobiochemistry |journal=] |volume=11 |pages=389–394 |doi=10.2113/gselements.11.6.395}}</ref> and that the founding lineages of the archaea and bacteria were H<sub>2</sub>-dependent autotrophs that used CO<sub>2</sub> as their terminal acceptor in energy metabolism.<ref>{{cite journal |last1=Martin |first1=W. |last2=Russell |first2= M. J. |year=2007 |title=On the origin of biochemistry at an alkaline hydrothermal vent |journal=] |volume=362 |issue= 1486 |pages=1887–1925 |doi=10.1098/rstb.2006.1881 |pmid=17255002 |pmc=2442388}}</ref> In 2016, Martin suggested, based upon this evidence, that the LUCA "may have depended heavily on the geothermal energy of the vent to survive".<ref name="Weiss Sousa Mrnjavac 2016" /> Pores at deep sea hydrothermal vents are suggested to have been occupied by membrane-bound compartments which promoted biochemical reactions.<ref>{{cite journal |last1=Lane |first1=Nick |last2=Martin |first2=William F. |date=2012-12-21 |title=The Origin of Membrane Bioenergetics |journal=Cell |volume=151 |issue=7 |pages=1406–1416 |doi= 10.1016/j.cell.2012.11.050 |pmid=23260134 |s2cid=15028935 |doi-access=free }}</ref><ref>{{cite journal |last1=Baaske |first1=Philipp |last2= Weinert |first2=Franz M. |last3=Duhr |first3=Stefan |last4=Lemke |first4=Kono H. |last5=Russell |first5=Michael J. |last6=Braun |first6=Dieter |date=2007-05-29 |title=Extreme accumulation of nucleotides in simulated hydrothermal pore systems |journal=Proceedings of the National Academy of Sciences |volume=104 |issue=22 |pages= 9346–9351 |doi=10.1073/pnas.0609592104 |pmc=1890497 |pmid=17494767 |doi-access=free|bibcode=2007PNAS..104.9346B }}</ref> Metabolic intermediates in the Krebs cycle, gluconeogenesis, amino acid bio-synthetic pathways, glycolysis, the pentose phosphate pathway, and including sugars like ribose, and lipid precursors can occur non-enzymatically at conditions relevant to deep-sea alkaline hydrothermal vents.<ref>{{Cite journal |last1=Nunes Palmeira |first1=Raquel |last2=Colnaghi |first2=Marco |last3=Harrison |first3=Stuart A. |last4=Pomiankowski |first4=Andrew |last5=Lane |first5=Nick |author5-link=Nick Lane |date=2022-11-09 |title=The limits of metabolic heredity in protocells |journal=Proceedings of the Royal Society B: Biological Sciences |volume=289 |issue=1986 |doi=10.1098/rspb.2022.1469 |pmc=9653231 |pmid=36350219}}</ref> | |||
| If the deep marine hydrothermal setting was the site for the origin of life, then abiogenesis could have happened as early as 4.0-4.2 Gya. If life evolved in the ocean at depths of more than ten meters, it would have been shielded both from impacts and the then high levels of ultraviolet radiation from the sun. The available energy in hydrothermal vents is maximized at 100–150 °C, the temperatures at which ] bacteria and ] ] live.<ref>{{Cite journal |last=Woese |first=Carl R. |date=1987 |title=Bacterial evolution |journal=Microbiological Reviews |volume=51.2 (1987) |issue=2 |pages=221–271 |doi=10.1128/mr.51.2.221-271.1987 |pmid=2439888 |pmc=373105 |s2cid=734579 }}</ref><ref>{{Citation |last1=Russell |first1=Michael J. |title=The onset and early evolution of life |date=2006 |work=Evolution of Early Earth's Atmosphere, Hydrosphere, and Biosphere - Constraints from Ore Deposits |publisher=Geological Society of America |last2=Hall |first2=Allan J. |doi=10.1130/2006.1198(01) |doi-broken-date=11 December 2024 |isbn=9780813711980 |url=https://pubs.geoscienceworld.org/gsa/books/book/442/chapter-abstract/3799005/The-onset-and-early-evolution-of-life?redirectedFrom=fulltext |archive-date=31 January 2024 |archive-url=https://web.archive.org/web/20240131155448/https://pubs.geoscienceworld.org/gsa/books/book/442/chapter-abstract/3799005/The-onset-and-early-evolution-of-life?redirectedFrom=fulltext |url-status=live }}</ref> Arguments against a hydrothermal origin of life state that hyperthermophily was a result of ] in bacteria and archaea, and that a ] environment would have been more likely.<ref>{{Cite journal |last1=Boussau |first1=Bastien |last2=Blanquart |first2=Samuel |last3=Necsulea |first3=Anamaria |last4=Lartillot |first4=Nicolas |last5=Gouy |first5=Manolo |date=December 2008 |title=Parallel adaptations to high temperatures in the Archaean eon |url=https://www.nature.com/articles/nature07393 |journal=Nature |volume=456 |issue=7224 |pages=942–945 |doi=10.1038/nature07393 |pmid=19037246 |bibcode=2008Natur.456..942B |s2cid=4348746 |access-date=8 December 2023 |archive-date=16 December 2023 |archive-url=https://web.archive.org/web/20231216095135/https://www.nature.com/articles/nature07393 |url-status=live }}</ref><ref name="science.org">{{Cite journal |last1=Galtier |first1=Nicolas |last2=Tourasse |first2=Nicolas |last3=Gouy |first3=Manolo |date=1999-01-08 |title=A Nonhyperthermophilic Common Ancestor to Extant Life Forms |url=https://www.science.org/doi/10.1126/science.283.5399.220 |journal=Science |volume=283 |issue=5399 |pages=220–221 |doi=10.1126/science.283.5399.220 |pmid=9880254 |access-date=8 December 2023 |archive-date=31 January 2024 |archive-url=https://web.archive.org/web/20240131155324/https://www.science.org/doi/10.1126/science.283.5399.220 |url-status=live }}</ref> This hypothesis, suggested in 1999 by Galtier, was proposed one year before the discovery of the Lost City Hydrothermal Field, where white-smoker hydrothermal vents average ~45-90 °C.<ref>{{Cite journal |last1=Kelley |first1=Deborah S. |last2=Karson |first2=Jeffrey A. |last3=Blackman |first3=Donna K. |last4=Früh-Green |first4=Gretchen L. |last5=Butterfield |first5=David A. |last6=Lilley |first6=Marvin D. |last7=Olson |first7=Eric J. |last8=Schrenk |first8=Matthew O. |last9=Roe |first9=Kevin K. |last10=Lebon |first10=Geoff T. |last11=Rivizzigno |first11=Pete |date=July 2001 |title=An off-axis hydrothermal vent field near the Mid-Atlantic Ridge at 30° N |journal=Nature |volume=412 |issue=6843 |pages=145–149 |doi=10.1038/35084000 |pmid=11449263 |bibcode=2001Natur.412..145K |s2cid=4407013 |url=https://www.nature.com/articles/35084000 |archive-date=31 January 2024 |archive-url=https://web.archive.org/web/20240131155327/https://www.nature.com/articles/35084000 |url-status=live }}</ref> Moderate temperatures and alkaline seawater such as that at Lost City are now the favoured hydrothermal vent setting in contrast to acidic, high temperature (~350 °C) black-smokers. | |||
| ==== Arguments against a vent setting ==== | |||
| Production of prebiotic organic compounds at hydrothermal vents is estimated to be 1x10<sup>8</sup> kg yr<sup>−1</sup>.<sup><ref name="Ehrenfreund Irvine Becker 2002">{{Cite journal |last1=Ehrenfreund |first1=P. |last2=Irvine |first2=W. |last3=Becker |first3=L. |last4=Blank |first4=J. |last5=Brucato |first5=J. R. |last6=Colangeli |first6=L. |last7=Derenne |first7=S. |last8=Despois |first8=D. |last9=Dutrey |first9=A. |last10=Fraaije |first10=H. |last11=Lazcano |first11=A. |last12=Owen |first12=T. |last13=Robert |first13=F. |display-authors=5 |date=August 2002 |title=Astrophysical and astrochemical insights into the origin of life |journal=Reports on Progress in Physics |volume=65 |issue=10 |pages=1427 |doi=10.1088/0034-4885/65/10/202 |bibcode=2002RPPh...65.1427E |s2cid=250904448}}</ref></sup> While a large amount of key prebiotic compounds, such as methane, are found at vents, they are in far lower concentrations than estimates of a Miller-Urey Experiment environment. In the case of methane, the production rate at vents is around 2-4 orders of magnitude lower than predicted amounts in a ] surface atmosphere.<ref name="Ehrenfreund Irvine Becker 2002"/><ref>{{cite book |last1=Chyba |first1=C.F. |chapter=Comets and Prebiotic Organic Molecules on Early Earth |title=Comets and the Origin and Evolution of Life |pages=169–206 |publisher=Springer Berlin Heidelberg |isbn=978-3-540-33086-8 |last2=Chyba |first2=C.F. |last3=Hand |first3=K.P. |series=Advances in Astrobiology and Biogeophysics |date=2006 |doi=10.1007/3-540-33088-7_6 |chapter-url=https://link.springer.com/chapter/10.1007/3-540-33088-7_6 |archive-date=31 January 2024 |archive-url=https://web.archive.org/web/20240131155457/https://link.springer.com/chapter/10.1007/3-540-33088-7_6 |url-status=live }}</ref> | |||
| Other arguments against an oceanic vent setting for the origin of life include the inability to concentrate prebiotic materials due to strong dilution from seawater. This open-system cycles compounds through minerals that make up vents, leaving little residence time to accumulate.<ref>{{Citation |last=Chatterjee |first=Sankar |title=The Cradle of Life |date=2023 |work=From Stardust to First Cells: The Origin and Evolution of Early Life |pages=43–66 |editor-last=Chatterjee |editor-first=Sankar |place=Cham |publisher=Springer International Publishing |doi=10.1007/978-3-031-23397-5_6 |isbn=978-3-031-23397-5 |url=https://link.springer.com/chapter/10.1007/978-3-031-23397-5_6 |archive-date=31 January 2024 |archive-url=https://web.archive.org/web/20240131155449/https://link.springer.com/chapter/10.1007/978-3-031-23397-5_6 |url-status=live }}</ref> All modern cells rely on phosphates and potassium for nucleotide backbone and protein formation respectively, making it likely that the first life forms also shared these functions. These elements were not available in high quantities in the Archaean oceans as both primarily come from the weathering of continental rocks on land, far from vent settings. Submarine hydrothermal vents are not conducive to condensation reactions needed for polymerisation to form macromolecules.<ref>{{cite book |last=Deamer |first=David W. |chapter=Prospects for Life on Other Planets |date=2019-02-07 |title=Assembling Life |publisher=] |doi=10.1093/oso/9780190646387.003.0017 |isbn=978-0-19-064638-7 |url=https://academic.oup.com/book/40676/chapter-abstract/348367627?redirectedFrom=fulltext |archive-date=31 January 2024 |archive-url=https://web.archive.org/web/20240131155333/https://academic.oup.com/book/40676/chapter-abstract/348367627?redirectedFrom=fulltext |url-status=live }}</ref><ref>{{Cite journal |last1=Pearce |first1=Ben K. D. |last2=Pudritz |first2=Ralph E. |last3=Semenov |first3=Dmitry A. |last4=Henning |first4=Thomas K. |date=2017-10-02 |title=Origin of the RNA world: The fate of nucleobases in warm little ponds |journal=Proceedings of the National Academy of Sciences |volume=114 |issue=43 |pages=11327–11332 |doi=10.1073/pnas.1710339114 |pmid=28973920 |pmc=5664528 |bibcode=2017PNAS..11411327P |doi-access=free |arxiv=1710.00434 }}</ref> | |||
| An older argument was that key polymers were encapsulated in vesicles after condensation, which supposedly would not happen in saltwater because of the high concentrations of ions. However, while it is true that salinity inhibits vesicle formation from low-diversity mixtures of fatty acids,<ref name="Deamer 2021"/> vesicle formation from a broader, more realistic mix of fatty-acid and 1-alkanol species is more resilient.<ref name="Jordan 2019">{{cite journal|last1=Jordan|first1=Sean F.|last2=Rammu|first2=Hanadi|last3=Zheludev|first3=Ivan N.|last4=Hartley|first4=Andrew M.|last5=Maréchal|first5=Amandine|last6=Lane|first6=Nick|date=2019|title=Promotion of protocell self-assembly from mixed amphiphiles at the origin of life|url=https://www.nature.com/articles/s41559-019-1015-y|journal=Nature Ecology & Evolution|volume=3|issue=12|pages=1705–1714|doi=10.1038/s41559-019-1015-y|pmid=31686020 |bibcode=2019NatEE...3.1705J }}</ref><ref name="Deamer 2021"/> | |||
| === Surface bodies of water === | |||
| Surface bodies of water provide environments able to dry out and be rewetted. Continued wet-dry cycles allow the concentration of prebiotic compounds and ]s to polymerise macromolecules. Moreover, lake and ponds on land allow for detrital input from the weathering of continental rocks which contain ], the most common source of phosphates needed for nucleotide backbones. The amount of exposed continental crust in the Hadean is unknown, but models of early ocean depths and rates of ocean island and continental crust growth make it plausible that there was exposed land.<ref>{{Cite journal |last=Korenaga |first=Jun |date=November 2021 |title=Was There Land on the Early Earth? |journal=Life |volume=11 |issue=11 |pages=1142 |doi=10.3390/life11111142 |pmc=8623345 |pmid=34833018 |bibcode=2021Life...11.1142K |doi-access=free}}</ref> Another line of evidence for a surface start to life is the requirement for ] for organism function. UV is necessary for the formation of the U+C nucleotide ] by partial ] and nucleobase loss.<ref>{{Cite journal |last1=Powner |first1=Matthew W. |last2=Gerland |first2=Béatrice |last3=Sutherland |first3=John D. |date=May 2009 |title=Synthesis of activated pyrimidine ribonucleotides in prebiotically plausible conditions |url=https://www.nature.com/articles/nature08013 |journal=Nature |volume=459 |issue=7244 |pages=239–242 |doi=10.1038/nature08013 |pmid=19444213 |bibcode=2009Natur.459..239P |s2cid=4412117 |access-date=8 December 2023 |archive-date=12 November 2023 |archive-url=https://web.archive.org/web/20231112211237/https://www.nature.com/articles/nature08013 |url-status=live }}</ref> Simultaneously, UV can be harmful and sterilising to life, especially for simple early lifeforms with little ability to repair radiation damage. Radiation levels from a young Sun were likely greater, and, with no ], harmful shortwave UV rays would reach the surface of Earth. For life to begin, a shielded environment with influx from UV-exposed sources is necessary to both benefit and protect from UV. Shielding under ice, liquid water, mineral surfaces (e.g. clay) or regolith is possible in a range of surface water settings. While deep sea vents may have input from raining down of surface exposed materials, the likelihood of concentration is lessened by the ocean's open system.<ref>{{Cite journal |last1=Zahnle |first1=Kevin |last2=Arndt |first2=Nick |last3=Cockell |first3=Charles |last4=Halliday |first4=Alex |last5=Nisbet |first5=Euan |last6=Selsis |first6=Franck |last7=Sleep |first7=Norman H. |date=2007-03-01 |title=Emergence of a Habitable Planet |journal=Space Science Reviews |volume=129 |issue=1 |pages=35–78 |doi=10.1007/s11214-007-9225-z |bibcode=2007SSRv..129...35Z |s2cid=12006144 |url=https://link.springer.com/article/10.1007/s11214-007-9225-z |archive-date=31 January 2024 |archive-url=https://web.archive.org/web/20240131155452/https://link.springer.com/article/10.1007/s11214-007-9225-z |url-status=live }}</ref> | |||
| ==== Hot springs ==== | |||
| Most branching phylogenies are thermophilic or hyperthermophilic, making it possible that the ] (LUCA) and preceding lifeforms were similarly thermophilic. Hot springs are formed from the heating of groundwater by geothermal activity. This intersection allows for influxes of material from deep penetrating waters and from surface runoff that transports eroded continental sediments. Interconnected groundwater systems create a mechanism for distribution of life to wider area.<ref>{{Cite journal |last=Woese |first=C R |date=June 1987 |title=Bacterial evolution |journal=Microbiological Reviews |volume=51 |issue=2 |pages=221–271 |doi=10.1128/mr.51.2.221-271.1987 |pmc=373105 |pmid=2439888}}</ref> | |||
| Mulkidjanian and co-authors argue that marine environments did not provide the ionic balance and composition universally found in cells, or the ions required by essential proteins and ribozymes, especially with respect to high K<sup>+</sup>/Na<sup>+</sup> ratio, Mn<sup>2+</sup>, Zn<sup>2+</sup> and phosphate concentrations. They argue that the only environments that mimic the needed conditions on Earth are hot springs similar to ones at Kamchatka.<ref name="Mulkidjanian Bychkov 2012">{{cite journal |last1=Mulkidjanian |first1=Armen Y. |last2=Bychkov |first2=Andrew Yu. |last3=Dibrova |first3=Daria V. |last4=Galperin |first4=Michael Y. |last5=Koonin |first5=Eugene V. |date=2012-04-03 |title=Origin of first cells at terrestrial, anoxic geothermal fields |journal=Proceedings of the National Academy of Sciences |volume=109 |issue=14 |pages=E821-30 |doi=10.1073/pnas.1117774109 |pmc=3325685 |pmid=22331915 |bibcode=2012PNAS..109E.821M |doi-access=free}}</ref> Mineral deposits in these environments under an anoxic atmosphere would have suitable pH (while current pools in an oxygenated atmosphere would not), contain precipitates of photocatalytic sulfide minerals that absorb harmful ultraviolet radiation, have wet-dry cycles that concentrate substrate solutions to concentrations amenable to spontaneous formation of biopolymers<ref>{{cite journal |last1=Chandru |first1=Kuhan |last2=Guttenberg |first2=Nicholas |last3=Giri |first3=Chaitanya |last4=Hongo |first4=Yayoi |last5=Butch |first5=Christopher |last6=Mamajanov |first6=Irena |last7=Cleaves |first7=H. James |display-authors=3 |title=Simple prebiotic synthesis of high diversity dynamic combinatorial polyester libraries |journal=] |date=31 May 2018 |volume=1 |issue=1 |page=30 |doi=10.1038/s42004-018-0031-1 |doi-access=free|bibcode=2018CmChe...1...30C }}</ref><ref>{{cite journal |last1=Forsythe |first1=Jay G. |last2=Yu |first2=Sheng-Sheng |last3=Mamajanov |first3=Irena |last4=Grover |first4=Martha A |author-link4=Martha Grover |last5=Krishnamurthy |first5=Ramanarayanan |last6=Fernández |first6=Facundo M. |last7=Hud |first7=Nicholas V. |display-authors=3 |date=17 August 2015 |title=Ester-Mediated Amide Bond Formation Driven by Wet–Dry Cycles: A Possible Path to Polypeptides on the Prebiotic Earth |journal=Angewandte Chemie International Edition in English |volume=54 |issue=34 |pages=9871–9875 |doi=10.1002/anie.201503792 |pmc=4678426 |pmid=26201989|bibcode=2015AngCh..54.9871F }}</ref> created both by chemical reactions in the hydrothermal environment, and by exposure to ] during transport from vents to adjacent pools that would promote the formation of biomolecules.<ref>{{cite journal |last1=Patel |first1=Bhavesh H. |last2=Percivalle |first2=Claudia |last3=Ritson |first3=Dougal J. |last4=Duffy |first4=Colm. D. |last5=Sutherland |first5=John D. |date=March 16, 2015 |title=Common origins of RNA, protein and lipid precursors in a cyanosulfidic protometabolism |journal=Nature Chemistry |volume=7 |issue=4 |pages=301–307 |doi=10.1038/nchem.2202 |pmc=4568310 |pmid=25803468 |bibcode=2015NatCh...7..301P}}</ref> The hypothesized pre-biotic environments are similar to hydrothermal vents, with additional components that help explain peculiarities of the LUCA.<ref name="Mulkidjanian Bychkov 2012"/><ref name="Damer 2020"/> | |||
| A phylogenomic and geochemical analysis of proteins plausibly traced to the LUCA shows that the ionic composition of its intracellular fluid is identical to that of hot springs. The LUCA likely was dependent upon synthesized organic matter for its growth.<ref name="Mulkidjanian Bychkov 2012"/> Experiments show that RNA-like polymers can be synthesized in wet-dry cycling and UV light exposure. These polymers were encapsulated in vesicles after condensation.<ref name="Deamer 2021">{{cite journal |last=Deamer |first=David |author-link=David W. Deamer |date=10 February 2021 |title=Where Did Life Begin? Testing Ideas in Prebiotic Analogue Conditions |journal=] |volume=11 |issue=2 |page=134 |doi=10.3390/life11020134 |pmid=33578711 |pmc=7916457 |bibcode=2021Life...11..134D |doi-access=free}}</ref> Potential sources of organics at hot springs might have been transport by interplanetary dust particles, extraterrestrial projectiles, or atmospheric or geochemical synthesis. Hot springs could have been abundant in volcanic landmasses during the Hadean.<ref name="Damer 2020"/> | |||
| ==== Temperate surface bodies of water ==== | |||
| A ] start in surface bodies of waters hypothesis has evolved from Darwin's concept of a ']' and the ]. Freshwater bodies under temperate climates can accumulate prebiotic materials while providing suitable environmental conditions conducive to simple life forms. The climate during the Archaean is still a highly debated topic, as there is uncertainty about what continents, oceans, and the atmosphere looked like then. Atmospheric reconstructions of the Archaean from geochemical proxies and models state that sufficient greenhouse gases were present to maintain surface temperatures between 0-40 °C. Under this assumption, there is a greater abundance of moderate temperature niches in which life could begin.<ref>{{Cite journal |last1=Catling |first1=David C. |last2=Zahnle |first2=Kevin J. |date=2020-02-28 |title=The Archean atmosphere |journal=Science Advances |volume=6 |issue=9 |pages=eaax1420 |doi=10.1126/sciadv.aax1420 |pmc=7043912 |pmid=32133393|bibcode=2020SciA....6.1420C }}</ref> | |||
| Strong lines of evidence for mesophily from biomolecular studies include Galtier's ] nucleotide thermometer. G+C are more abundant in thermophiles due to the added stability of an additional hydrogen bond not present between A+T nucleotides. ] sequencing on a diverse range of modern lifeforms show that ]'s reconstructed G+C content was likely representative of moderate temperatures.<ref name="science.org"/> | |||
| Although most modern phylogenies are thermophilic or hyperthermophilic<!--REPETITION-->, it is possible that their widespread diversity today is a product of convergent evolution and horizontal gene transfer rather than an inherited trait from LUCA.<ref>{{Cite journal |last1=Miller |first1=Stanley L. |last2=Lazcano |first2=Antonio |date=December 1995 |title=The origin of life?did it occur at high temperatures? |journal=Journal of Molecular Evolution |volume=41 |issue=6 |pages=689–692 |doi=10.1007/bf00173146 |pmid=11539558 |bibcode=1995JMolE..41..689M |s2cid=25141419 |hdl=2060/19980211388 |url=https://link.springer.com/article/10.1007/BF00173146 |hdl-access=free |archive-date=31 January 2024 |archive-url=https://web.archive.org/web/20240131155843/https://link.springer.com/article/10.1007/BF00173146 |url-status=live }}</ref> The ] ] is found exclusively in thermophiles and hyperthermophiles as it allows for coiling of DNA.<ref>{{Cite journal |last1=Forterre |first1=Patrick |last2=Bergerat |first2=Agnes |last3=Lopex-Garcia |first3=Purificacion |date=May 1996 |title=The unique DNA topology and DNA topoisomerases of hyperthermophilic archaea |journal=FEMS Microbiology Reviews |volume=18 |issue=2–3 |pages=237–248 |doi=10.1111/j.1574-6976.1996.tb00240.x |pmid=8639331 |s2cid=6001830|doi-access=free }}</ref> The reverse gyrase enzyme requires ] to function, both of which are complex biomolecules. If an origin of life is hypothesised to involve a simple organism that had not yet evolved a membrane, let alone ATP, this would make the existence of reverse gyrase improbable. Moreover, phylogenetic studies show that reverse gyrase had an archaeal origin, and that it was transferred to bacteria by horizontal gene transfer. This implies that reverse gyrase was not present in the LUCA.<ref>{{Cite journal |last1=Brochier-Armanet |first1=Céline |last2=Forterre |first2=Patrick |date=May 2006 |title=Widespread distribution of archaeal reverse gyrase in thermophilic bacteria suggests a complex history of vertical inheritance and lateral gene transfers |journal=Archaea |volume=2 |issue=2 |pages=83–93 |doi=10.1155/2006/582916 |pmid=17350929 |pmc=2686386 |doi-access=free}}</ref> | |||
| ==== Icy surface bodies of water ==== | |||
| Cold-start origin of life theories stem from the idea there may have been cold enough regions on the early Earth that large ice cover could be found. Stellar evolution models predict that the Sun's luminosity was ~25% weaker than it is today. Fuelner states that although this significant decrease in solar energy would have formed an icy planet, there is strong evidence for liquid water to be present, possibly driven by a greenhouse effect. This would create an early Earth with both liquid oceans and icy poles.<ref>{{Cite journal |last=Feulner |first=Georg |date=June 2012 |title=The faint young Sun problem |url=https://agupubs.onlinelibrary.wiley.com/doi/10.1029/2011RG000375 |journal=Reviews of Geophysics |volume=50 |issue=2 |doi=10.1029/2011RG000375 |arxiv=1204.4449 |bibcode=2012RvGeo..50.2006F |s2cid=119248267 |access-date=8 December 2023 |archive-date=8 December 2023 |archive-url=https://web.archive.org/web/20231208060325/https://agupubs.onlinelibrary.wiley.com/doi/10.1029/2011RG000375 |url-status=live }}</ref> | |||
| Ice melts that form from ice sheets or glaciers melts create freshwater pools, another niche capable of experiencing wet-dry cycles. While these pools that exist on the surface would be exposed to intense UV radiation, bodies of water within and under ice are sufficiently shielded while remaining connected to UV exposed areas through ice cracks. Suggestions of impact melting of ice allow freshwater paired with meteoritic input, a popular vessel for prebiotic components.<ref>{{Cite journal |last1=Bada |first1=J. L. |last2=Bigham |first2=C. |last3=Miller |first3=S. L. |date=1994-02-15 |title=Impact melting of frozen oceans on the early Earth: Implications for the origin of life |journal=Proceedings of the National Academy of Sciences |volume=91 |issue=4 |pages=1248–1250 |doi=10.1073/pnas.91.4.1248 |pmc=43134 |pmid=11539550 |bibcode=1994PNAS...91.1248B |doi-access=free}}</ref> Near-seawater levels of sodium chloride are found to destabilize fatty acid membrane self-assembly, making freshwater settings appealing for early membranous life.<ref>{{Cite journal |last1=Monnard |first1=Pierre-Alain |last2=Apel |first2=Charles L. |last3=Kanavarioti |first3=Anastassia |last4=Deamer |first4=David W. |date=June 2002 |title=Influence of Ionic Inorganic Solutes on Self-Assembly and Polymerization Processes Related to Early Forms of Life: Implications for a Prebiotic Aqueous Medium |url=https://www.liebertpub.com/doi/10.1089/15311070260192237 |journal=Astrobiology |volume=2 |issue=2 |pages=139–152 |doi=10.1089/15311070260192237 |pmid=12469365 |bibcode=2002AsBio...2..139M |access-date=8 December 2023 |archive-date=31 January 2024 |archive-url=https://web.archive.org/web/20240131155030/https://www.liebertpub.com/doi/10.1089/15311070260192237 |url-status=live }}</ref> | |||
| Icy environments would trade the faster reaction rates that occur in warm environments for increased stability and accumulation of larger polymers.<ref>{{Cite journal |last1=Attwater |first1=James |last2=Wochner |first2=Aniela |last3=Holliger |first3=Philipp |date=December 2013 |title=In-ice evolution of RNA polymerase ribozyme activity |journal=Nature Chemistry |volume=5 |issue=12 |pages=1011–1018 |doi=10.1038/nchem.1781 |pmid=24256864 |pmc=3920166|bibcode=2013NatCh...5.1011A }}</ref> Experiments simulating Europa-like conditions of ~20 °C have synthesised amino acids and adenine, showing that Miller-Urey type syntheses can still occur at cold temperatures.<ref>{{Cite journal |last1=Levy |first1=Matthew |last2=Miller |first2=Stanley L. |last3=Brinton |first3=Karen |last4=Bada |first4=Jeffrey L. |date=2000-06-01 |title=Prebiotic Synthesis of Adenine and Amino Acids Under Europa-like Conditions |url=https://www.sciencedirect.com/science/article/pii/S0019103500963656 |journal=Icarus |volume=145 |issue=2 |pages=609–613 |doi=10.1006/icar.2000.6365|pmid=11543508 |bibcode=2000Icar..145..609L }}</ref> In an ], the ribozyme would have had even more functions than in a later DNA-RNA-protein-world. For RNA to function, it must be able to fold, a process that is hindered by temperatures above 30 °C. While RNA folding in ] organisms is slower, the process is more successful as hydrolysis is also slower. Shorter nucleotides would not suffer from higher temperatures.<ref>{{Cite journal |last1=Moulton |first1=Vincent |last2=Gardner |first2=Paul P. |last3=Pointon |first3=Robert F. |last4=Creamer |first4=Lawrence K. |last5=Jameson |first5=Geoffrey B. |last6=Penny |first6=David |date=2000-10-01 |title=RNA Folding Argues Against a Hot-Start Origin of Life |journal=Journal of Molecular Evolution |volume=51 |issue=4 |pages=416–421 |doi=10.1007/s002390010104 |pmid=11040293 |bibcode=2000JMolE..51..416M |s2cid=20787323 |url=https://link.springer.com/article/10.1007/s002390010104 |archive-date=31 January 2024 |archive-url=https://web.archive.org/web/20240131155950/https://link.springer.com/article/10.1007/s002390010104 |url-status=live }}</ref><ref>{{Cite journal |last1=Zemora |first1=Georgeta |last2=Waldsich |first2=Christina |date=November 2010 |title=RNA folding in living cells |journal=RNA Biology |volume=7 |issue=6 |pages=634–641 |doi=10.4161/rna.7.6.13554 |pmid=21045541 |pmc=3073324 }}</ref> | |||
| === Inside the continental crust === | |||
| An alternative geological environment has been proposed by the geologist Ulrich Schreiber and the physical chemist Christian Mayer: the ].<ref>{{Cite journal |last1=Schreiber |first1=Ulrich |last2=Locker-Grütjen |first2=Oliver |last3=Mayer |first3=Christian |date=2012 |title=Hypothesis: Origin of Life in Deep-Reaching Tectonic Faults |url=https://link.springer.com/10.1007/s11084-012-9267-4 |journal=Origins of Life and Evolution of Biospheres |language=en |volume=42 |issue=1 |pages=47–54 |doi=10.1007/s11084-012-9267-4 |pmid=22373604 |bibcode=2012OLEB...42...47S |issn=0169-6149}}</ref> ] zones could present a stable and well-protected environment for long-term prebiotic evolution. Inside these systems of cracks and cavities, water and carbon dioxide present the bulk solvents. Their phase state would depend on the local temperature and pressure conditions and could vary between liquid, gaseous and ]. When forming two separate phases (e.g., liquid water and supercritical carbon dioxide in depths of little more than 1 km), the system provides optimal conditions for ]. Concurrently, the contents of the tectonic fault zones are being supplied by a multitude of inorganic educts (e.g., carbon monoxide, hydrogen, ammonia, hydrogen cyanide, nitrogen, and even phosphate from dissolved apatite) and simple organic molecules formed by hydrothermal chemistry (e.g. amino acids, long-chain amines, fatty acids, long-chain aldehydes).<ref>{{Cite journal |last1=Schreiber |first1=Ulrich |last2=Mayer |first2=Christian |last3=Schmitz |first3=Oliver J. |last4=Rosendahl |first4=Pia |last5=Bronja |first5=Amela |last6=Greule |first6=Markus |last7=Keppler |first7=Frank |last8=Mulder |first8=Ines |last9=Sattler |first9=Tobias |last10=Schöler |first10=Heinz F. |date=2017-06-14 |editor-last=Stüeken |editor-first=Eva Elisabeth |title=Organic compounds in fluid inclusions of Archean quartz—Analogues of prebiotic chemistry on early Earth |journal=PLOS ONE |language=en |volume=12 |issue=6 |pages=e0177570 |doi=10.1371/journal.pone.0177570 |doi-access=free |issn=1932-6203 |pmc=5470662 |pmid=28614348|bibcode=2017PLoSO..1277570S }}</ref><ref>{{Cite journal |last1=Großmann |first1=Yildiz |last2=Schreiber |first2=Ulrich |last3=Mayer |first3=Christian |last4=Schmitz |first4=Oliver J. |date=2022-06-21 |title=Aliphatic Aldehydes in the Earth's Crust—Remains of Prebiotic Chemistry? |journal=Life |language=en |volume=12 |issue=7 |pages=925 |doi=10.3390/life12070925 |doi-access=free |issn=2075-1729 |pmc=9319801 |pmid=35888015|bibcode=2022Life...12..925G }}</ref> Finally, the abundant mineral surfaces provide a rich choice of catalytic activity. | |||
| An especially interesting section of the tectonic fault zones is located at a depth of approximately 1000 m. For the carbon dioxide part of the bulk solvent, it provides temperature and pressure conditions near the ] point between the ] and the gaseous state. This leads to a natural accumulation zone for ] that dissolve well in ], but not in its gaseous state, leading to their local precipitation.<ref>{{Cite journal |last1=Mayer |first1=Christian |last2=Schreiber |first2=Ulrich |last3=Dávila |first3=María |date=2017-01-07 |title=Selection of Prebiotic Molecules in Amphiphilic Environments |journal=Life |language=en |volume=7 |issue=1 |pages=3 |doi=10.3390/life7010003 |doi-access=free |issn=2075-1729 |pmc=5370403 |pmid=28067845|bibcode=2017Life....7....3M }}</ref> Periodic pressure variations such as caused by ] or ] result in periodic phase transitions, keeping the local reaction environment in a constant ]. In presence of ] (such as the long chain amines and fatty acids mentioned above), subsequent generations of vesicles are being formed<ref>{{Cite journal |last1=Mayer |first1=Christian |last2=Schreiber |first2=Ulrich |last3=Dávila |first3=María J. |date=2015 |title=Periodic Vesicle Formation in Tectonic Fault Zones—an Ideal Scenario for Molecular Evolution |journal=Origins of Life and Evolution of Biospheres |language=en |volume=45 |issue=1–2 |pages=139–148 |doi=10.1007/s11084-015-9411-z |issn=0169-6149 |pmc=4457167 |pmid=25716918|bibcode=2015OLEB...45..139M }}</ref> that are constantly and efficiently being selected for their stability.<ref>{{Cite journal |last1=Mayer |first1=Christian |last2=Schreiber |first2=Ulrich |last3=Dávila |first3=María |last4=Schmitz |first4=Oliver |last5=Bronja |first5=Amela |last6=Meyer |first6=Martin |last7=Klein |first7=Julia |last8=Meckelmann |first8=Sven |date=2018-05-24 |title=Molecular Evolution in a Peptide-Vesicle System |journal=Life |language=en |volume=8 |issue=2 |pages=16 |doi=10.3390/life8020016 |doi-access=free |issn=2075-1729 |pmc=6027363 |pmid=29795023|bibcode=2018Life....8...16M }}</ref> The resulting structures could provide hydrothermal vents as well as hot springs with raw material for further development. | |||
| == Homochirality == | |||
| {{main|Homochirality}} | |||
| ], are ], and occur in living systems in only one of the two possible forms, in the case of ]s the left-handed form. Prebiotic chemistry would produce both forms, creating a puzzle for abiogenesis researchers.<ref name="Plasson2007"/>]] | |||
| Homochirality is the geometric uniformity of materials composed of ] (non-mirror-symmetric) units. Living organisms use molecules that have the same chirality (handedness): with almost no exceptions,<ref>{{harvnb|Chaichian|Rojas|Tureanu|2014|pp=353–364}}</ref> amino acids are left-handed while nucleotides and ] are right-handed. Chiral molecules can be synthesized, but in the absence of a chiral source or a chiral catalyst, they are formed in a 50/50 (racemic) mixture of both ]. Known mechanisms for the production of non-racemic mixtures from racemic starting materials include: asymmetric physical laws, such as the ]; asymmetric environments, such as those caused by ] light, ], or the Earth's rotation, ] during racemic synthesis,<ref name="Plasson2007">{{cite journal |last1=Plasson |first1=Raphaël |last2=Kondepudi |first2=Dilip K. |last3=Bersini |first3=Hugues |last4=Commeyras |first4=Auguste |last5=Asakura |first5=Kouichi |display-authors=3 |date=August 2007 |title=Emergence of homochirality in far-from-equilibrium systems: Mechanisms and role in prebiotic chemistry |journal=] |volume=19 |issue=8 |pages=589–600 |doi=10.1002/chir.20440 |pmid=17559107}} "Special Issue: Proceedings from the Eighteenth International Symposium on Chirality (ISCD-18), Busan, Korea, 2006"</ref> and ].<ref name="jafarpour2017">{{cite journal |last1=Jafarpour |first1=Farshid |last2=Biancalani |first2=Tommaso |last3=Goldenfeld |first3=Nigel |author-link3=Nigel Goldenfeld |year=2017 |title=Noise-induced symmetry breaking far from equilibrium and the emergence of biological homochirality |url=http://dspace.mit.edu/bitstream/1721.1/109170/1/PhysRevE.95.032407.pdf |journal=] |volume=95 |issue=3 |page=032407 |bibcode=2017PhRvE..95c2407J |doi=10.1103/PhysRevE.95.032407 |pmid=28415353 |doi-access=free |access-date=29 August 2019 |archive-date=2 April 2023 |archive-url=https://web.archive.org/web/20230402201812/http://dspace.mit.edu/bitstream/handle/1721.1/109170/PhysRevE.95.032407.pdf;jsessionid=14B762FE82E5B78CD32F99AEE6E1A5F5?sequence=1 |url-status=live }}</ref><ref name="jafarpour2015">{{cite journal |last1=Jafarpour |first1=Farshid |last2=Biancalani |first2=Tommaso |last3=Goldenfeld |first3=Nigel |author-link3=Nigel Goldenfeld |year=2015 |title=Noise-induced mechanism for biological homochirality of early life self-replicators |journal=] |volume=115 |issue=15 |page=158101 |arxiv=1507.00044 |bibcode=2015PhRvL.115o8101J |doi=10.1103/PhysRevLett.115.158101 |pmid=26550754 |s2cid=9775791}}</ref><ref name="frank1953">{{cite journal |last1=Frank |first1=F. C. |year=1953 |title=On spontaneous asymmetric synthesis |journal=] |volume=11 |issue=4 |pages=459–463 |doi=10.1016/0006-3002(53)90082-1 |pmid=13105666}}</ref> | |||
| Once established, chirality would be selected for.<ref>{{cite journal |last=Clark |first=Stuart |author-link=Stuart Clark (author) |date=July–August 1999 |title=Polarized Starlight and the Handedness of Life |journal=] |volume=87 |issue=4 |page=336 |bibcode=1999AmSci..87..336C |doi=10.1511/1999.30.336 |s2cid=221585816 }}</ref> A small bias (]) in the population can be amplified into a large one by ], such as in the ].<ref>{{cite journal |last1=Shibata |first1=Takanori |last2=Morioka |first2=Hiroshi |last3=Hayase |first3=Tadakatsu |last4=Choji |first4=Kaori |last5=Soai |first5=Kenso |author5-link=Kensō Soai |display-authors=3 |date=17 January 1996 |title=Highly Enantioselective Catalytic Asymmetric Automultiplication of Chiral Pyrimidyl Alcohol |journal=] |volume=118 |issue=2 |pages=471–472 |doi=10.1021/ja953066g|bibcode=1996JAChS.118..471S }}</ref> In asymmetric autocatalysis, the catalyst is a chiral molecule, which means that a chiral molecule is catalyzing its own production. An initial enantiomeric excess, such as can be produced by polarized light, then allows the more abundant enantiomer to outcompete the other.<ref name="Soai2001">{{cite journal |last1=Soai |first1=Kenso |last2=Sato |first2=Itaru |last3=Shibata |first3=Takanori |year=2001 |title=Asymmetric autocatalysis and the origin of chiral homogeneity in organic compounds |journal=The Chemical Record |volume=1 |issue=4 |pages=321–332 |doi=10.1002/tcr.1017 |pmid=11893072}}</ref> | |||
| Homochirality may have started in outer space, as on the ] the amino acid ] (left-handed) is more than twice as frequent as its D (right-handed) form, and ] is more than three times as abundant as its D counterpart.<ref>{{harvnb|Hazen|2005|p=184}}</ref><ref name="Meierhenrich">{{cite book |last1=Meierhenrich |first1=Uwe |title=Amino acids and the asymmetry of life caught in the act of formation |date=2008 |publisher=Springer |isbn=978-3-540-76886-9 |location=Berlin |pages=76–79}}</ref> Amino acids from meteorites show a left-handed bias, whereas sugars show a predominantly right-handed bias: this is the same preference found in living organisms, suggesting an abiogenic origin of these compounds.<ref name="StarStuff">{{cite journal |last=Mullen |first=Leslie |title=Building Life from Star-Stuff |journal=] |date=5 September 2005 |url=http://www.astrobio.net/news-exclusive/building-life-from-star-stuff/ |url-status=usurped |archive-url=https://web.archive.org/web/20150714084344/http://www.astrobio.net/news-exclusive/building-life-from-star-stuff/ |archive-date=14 July 2015}}</ref> | |||
| In a 2010 experiment by Robert Root-Bernstein, "two D-RNA-oligonucleotides having inverse base sequences (D-CGUA and D-AUGC) and their corresponding L-RNA-oligonucleotides (L-CGUA and L-AUGC) were synthesized and their affinity determined for Gly and eleven pairs of L- and D-amino acids". The results suggest that homochirality, including codon directionality, might have "emerged as a function of the origin of the genetic code".<ref>{{Cite journal |last=Root-Bernstein |first=Robert |date=23 June 2010 |title=Experimental Test of L- and D-Amino Acid Binding to L- and D-Codons Suggests that Homochirality and Codon Directionality Emerged with the Genetic Code |journal=Symmetry |volume=2 |issue=2 |pages=1180–1200 |doi=10.3390/sym2021180 |bibcode=2010Symm....2.1180R |doi-access=free }}</ref> | |||
| == See also == | |||
| * ] | |||
| * ] | |||
| * {{Annotated link|Formamide-based prebiotic chemistry}} | |||
| * {{Annotated link|Proto-metabolism}} | |||
| * {{Annotated link|GADV-protein world hypothesis}} | |||
| * {{Annotated link|Genetic recombination}} | |||
| * {{Annotated link|Shadow biosphere}} | |||
| * ] | |||
| == Notes == | |||
| {{notelist}} | |||
| == References == | |||
| {{reflist}} | |||
| == Sources == | |||
| {{Refbegin|colwidth=30em}} | |||
| * {{cite book |last=Altermann |first=Wladyslaw |year=2009 |chapter=Introduction: A Roadmap to Fata Morgana? |editor1-last=Seckbach |editor1-first=Joseph |editor2-last=Walsh |editor2-first=Maud |title=From Fossils to Astrobiology: Records of Life on Earth and the Search for Extraterrestrial Biosignatures |series=Cellular Origin, Life in Extreme Habitats and Astrobiology |volume=12 |location=Dordrecht, the Netherlands; London |publisher=Springer |isbn=978-1-4020-8836-0}} | |||
| * {{cite book |last1=Bada |first1=Jeffrey L. |author1-link=Jeffrey L. Bada |last2=Lazcano |first2=Antonio |author2-link=Antonio Lazcano |year=2009 |chapter=The Origin of Life |editor1-last=Ruse |editor1-first=Michael |editor1-link=Michael Ruse |editor2-last=Travis |editor2-first=Joseph |editor2-link=Joseph Travis |title=Evolution: The First Four Billion Years |others=Foreword by ] |location=Cambridge |publisher=] |isbn=978-0-674-03175-3 |oclc=225874308 |chapter-url=https://archive.org/details/evolutionfirstfo00mich }} | |||
| * {{cite book |last1=Barton |first1=Nicholas H. |author-link1=Nick Barton |last2=Briggs |first2=Derek E.G. |author-link2=Derek Briggs |last3=Eisen |first3=Jonathan A. |author-link3=Jonathan Eisen |last4=Goldstein |first4=David B. |last5=Patel |first5=Nipan H. |display-authors=3 |year=2007 |title=Evolution |location=Cold Spring Harbor, New York |publisher=Cold Spring Harbor Laboratory Press |isbn=978-0-87969-684-9 |oclc=86090399}} | |||
| * {{cite book |last=Bastian |first=H. Charlton |author-link=Henry Charlton Bastian |year=1871 |title=The Modes of Origin of Lowest Organisms |url=https://archive.org/details/modesoforiginofl00bast |location=London; New York |publisher=] |oclc=42959303 |access-date=2015-06-06 }} | |||
| * {{cite book |last=Bernal |first=J. D. |author-link=John Desmond Bernal |year=1951 |title=The Physical Basis of Life |location=London |publisher=] & Kegan Paul}} | |||
| * {{cite book |last=Bernal |first=J. D. |year=1960 |chapter=The Problem of Stages in Biopoesis |editor-last=Florkin |editor-first=M. |editor-link=Marcel Florkin |title=Aspects of the Origin of Life |chapter-url=https://archive.org/details/aspectsoforigino0000flor |chapter-url-access=registration |series=International Series of Monographs on Pure and Applied Biology |location=Oxford, UK; New York |publisher=] |isbn=978-1-4831-3587-8 |ref=none }} | |||
| * {{cite book |last=Bernal |first=J. D. |year=1967 |orig-date=Reprinted work by ] originally published 1924; Moscow: ] |title=The Origin of Life |url=https://archive.org/details/originoflife0000bern |url-access=registration |series=The Weidenfeld and Nicolson Natural History |others=Translation of Oparin by Ann Synge |location=London |publisher=] }} | |||
| * {{cite book |editor1-last=Bock |editor1-first=Gregory R. |editor2-last=Goode |editor2-first=Jamie A. |year=1996 |title=Evolution of Hydrothermal Ecosystems on Earth (and Mars?) |series=Ciba Foundation Symposium |volume=202 |location=Chichester, UK; New York |publisher=] |isbn=978-0-471-96509-1}} | |||
| * {{cite book |last=Bondeson |first=Jan |author-link=Jan Bondeson |year=1999 |title=The Feejee Mermaid and Other Essays in Natural and Unnatural History |location=Ithaca, NY |publisher=] |isbn=978-0-8014-3609-3}} | |||
| * {{cite book |last=Bryson |first=Bill |author-link=Bill Bryson |year=2004 |title=A Short History of Nearly Everything |location=London |publisher=] |isbn=978-0-552-99704-1 |oclc=55589795 |title-link=A Short History of Nearly Everything}} | |||
| * {{cite book |last=Calvin |first=Melvin |author-link=Melvin Calvin |year=1969 |title=Chemical Evolution: Molecular Evolution Towards the Origin of Living Systems on the Earth and Elsewhere |url=https://archive.org/details/chemicalevolutio0000calv |url-access=registration |location=Oxford, UK |publisher=] |isbn=978-0-19-855342-7 |oclc=25220 }} | |||
| * {{cite book |last1=Chaichian |first1=Masud |last2=Rojas |first2=Hugo Perez |last3=Tureanu |first3=Anca |title=Basic Concepts in Physics |year=2014 |chapter=Physics and Life |series=Undergraduate Lecture Notes in Physics |pages=353–364 |location=Berlin; Heidelberg |publisher=] |doi=10.1007/978-3-642-19598-3_12 |isbn=978-3-642-19597-6 |s2cid=115247432 |oclc=900189038}} | |||
| * {{cite book |last=Chang |first=Thomas Ming Swi |author-link=Thomas Chang |year=2007 |title=Artificial Cells: Biotechnology, Nanomedicine, Regenerative Medicine, Blood Substitutes, Bioencapsulation, and Cell/Stem Cell Therapy |series=Regenerative Medicine, Artificial Cells and Nanomedicine |volume=1 |location=Hackensack, New Jersey |publisher=] |isbn=978-981-270-576-1 |oclc=173522612}} | |||
| * {{cite book |last=Dalrymple |first=G. Brent |author-link=Brent Dalrymple |year=2001 |chapter=The age of the Earth in the twentieth century: a problem (mostly) solved |editor1-last=Lewis |editor1-first=C.L.E. |editor2-last=Knell |editor2-first=S.J. |title=The Age of the Earth: from 4004 BC to AD 2002 |series=Geological Society Special Publication |location=London |publisher=] |volume=190 |issue=1 |pages=205–221 |bibcode=2001GSLSP.190..205D |doi=10.1144/gsl.sp.2001.190.01.14 |isbn=978-1-86239-093-5 |s2cid=130092094 |oclc=48570033}} | |||
| * {{cite book |last=Darwin |first=Charles |author-link=Charles Darwin |year=1887 |editor-last=Darwin |editor-first=Francis |editor-link=Francis Darwin |title=The Life and Letters of Charles Darwin, Including an Autobiographical Chapter |volume=3 |edition=3rd |location=London |publisher=] |oclc=834491774 |title-link=The Life and Letters of Charles Darwin}} | |||
| * {{cite book |last=Davies |first=Paul |author-link=Paul Davies |year=1999 |title=The Fifth Miracle: The Search for the Origin of Life |location=London |publisher=] |isbn=978-0-14-028226-9}} | |||
| * {{cite book |last=Dobell |first=Clifford |author-link=Clifford Dobell |title=Antony van Leeuwenhoek and His 'Little Animals' |url=https://archive.org/details/antonyvanleeuwen00clif |url-access=registration |year=1960 |orig-date=Originally published 1932; New York: ] |location=New York |publisher=] }} | |||
| * {{cite book |last=Dyson |first=Freeman |author-link=Freeman Dyson |year=1999 |title=Origins of Life |edition=Revised |location=Cambridge, UK; New York |publisher=] |isbn=978-0-521-62668-2 |ref=none}} | |||
| * {{cite book |last=Fesenkov |first=V. G. |author-link=Vasily Fesenkov |year=1959 |chapter=Some Considerations about the Primaeval State of the Earth |editor1-last=Oparin |editor1-first=A. I. |editor1-link=Alexander Oparin |editor2-last=Braunshtein |editor2-first=A.E. |editor3-last=Pasynskii |editor3-first=A. G. |editor4-last=Pavlovskaya |editor4-first=T. E. |display-editors=1 |title=The Origin of Life on the Earth |chapter-url=https://archive.org/details/proceedings00inte |series=I.U.B. Symposium Series |volume=1 |others=Edited for the ] by Frank Clark and ] |edition=English-French-German |location=London; New York |publisher=] |isbn=978-1-4832-2240-0 }} International Symposium on the Origin of Life on the Earth (held at Moscow, 19–24 August 1957) | |||
| * {{cite book |last=Hazen |first=Robert M. |author-link=Robert Hazen |title=Genesis: The Scientific Quest for Life's Origin |year=2005 |location=Washington, DC |publisher=] |isbn=978-0-309-09432-0 |oclc=60321860 |url-access=registration |url=https://archive.org/details/genesisscientifi0000haze }} | |||
| * {{cite book |last=Huxley |first=Thomas Henry |author-link=Thomas Henry Huxley |year=1968 |orig-date=1897 |chapter=VIII Biogenesis and Abiogenesis |chapter-url=http://aleph0.clarku.edu/huxley/CE8/B-Ab.html |title=Discourses, Biological and Geological |series=Collected Essays |volume=VIII |edition=Reprint |location=New York |publisher=] |oclc=476737627 |access-date=19 May 2014 |archive-date=7 May 2015 |archive-url=https://web.archive.org/web/20150507041155/http://aleph0.clarku.edu/huxley/CE8/B-Ab.html |url-status=live }} | |||
| * {{cite conference |url=http://www.panspermia.org/oseti.htm |title=Panspermia Asks New Questions |first=Brig |last=Klyce |date=22 January 2001 |conference=The Search for Extraterrestrial Intelligence (SETI) in the Optical Spectrum III |conference-url=http://www.coseti.org/4273-sch.htm |editor1-last=Kingsley |editor1-first=Stuart A. |editor1-link=Stuart Kingsley |editor2-last=Bhathal |editor2-first=Ragbir |editor2-link=Ragbir Bhathal |volume=4273 |publisher=] |location=Bellingham, WA |isbn=0-8194-3951-7 |doi=10.1117/12.435366 |access-date=2015-06-09 |archive-date=3 September 2013 |archive-url=https://web.archive.org/web/20130903122724/http://panspermia.org/oseti.htm |url-status=live }} Proceedings of the SPIE held at San Jose, California, 22–24 January 2001 | |||
| * {{cite book |last=Lane |first=Nick |author-link=Nick Lane |year=2009 |title=Life Ascending: The 10 Great Inventions of Evolution |edition=1st American |location=New York |publisher=W.W. Norton & Company |isbn=978-0-393-06596-1 |oclc=286488326 |url-access=registration |url=https://archive.org/details/lifeascendingten0000lane }} | |||
| * {{cite book |last=Lennox |first=James G. |author-link=James G. Lennox |year=2001 |title=Aristotle's Philosophy of Biology: Studies in the Origins of Life Science |series=Cambridge Studies in Philosophy and Biology |location=Cambridge, UK; New York |publisher=Cambridge University Press |isbn=978-0-521-65976-5}} | |||
| * {{cite book |last=Michod |first=Richard E. |year=1999 |title=Darwinian Dynamics: Evolutionary Transitions in Fitness and Individuality |location=Princeton, New Jersey |publisher=] |isbn=978-0-691-02699-2 |oclc=38948118 |url=https://archive.org/details/darwiniandynamic00mich }} | |||
| * {{cite book |last=Oparin |first=A.I. |author-link=Alexander Oparin |year=1953 |orig-date=Originally published 1938; New York: ] |title=The Origin of Life |others=Translation and new introduction by Sergius Morgulis |edition=2nd |location=Mineola, NY |publisher=Dover Publications |isbn=978-0-486-49522-4}} | |||
| * {{cite book |last=Ross |first=Alexander |author-link=Alexander Ross (writer) |year=1652 |title=Arcana Microcosmi |url=http://penelope.uchicago.edu/ross/ross210.html |volume=II |location=London |oclc=614453394 |access-date=14 July 2015 |archive-date=24 February 2024 |archive-url=https://web.archive.org/web/20240224160230/http://penelope.uchicago.edu/ross/ross210.html |url-status=live }} | |||
| * {{cite conference |chapter-url=http://www.rbsp.info/rbs/PDF/spie05-telos.pdf |chapter=Historical Development of the Distinction between Bio- and Abiogenesis |last=Sheldon |first=Robert B. |title=Astrobiology and Planetary Missions |date=22 September 2005 |conference=Astrobiology and Planetary Missions |conference-url=http://spie.org/Publications/Proceedings/Volume/5906?origin_id=x4323&start_year=2005&end_year=2005 |editor1-last=Hoover |editor1-first=Richard B. |editor1-link=Richard B. Hoover |editor2-last=Levin |editor2-first=Gilbert V. |editor2-link=Gilbert Levin |editor3-last=Rozanov |editor3-first=Alexei Y. |editor4-last=Gladstone |editor4-first=G. Randall |volume=5906 |pages=59061I |publisher=SPIE |location=Bellingham, WA |isbn=978-0-8194-5911-4 |doi=10.1117/12.663480 |access-date=13 April 2015 |archive-date=13 April 2015 |archive-url=https://web.archive.org/web/20150413020306/http://www.rbsp.info/rbs/PDF/spie05-telos.pdf |url-status=live }} Proceedings of the SPIE held at San Diego, California, 31 July–2 August 2005 | |||
| * {{cite book |last=Tyndall |first=John |author-link=John Tyndall |year=1905 |orig-date=Originally published 1871; London; New York: ]s, Green & Co.; ] |title=Fragments of Science |url=https://archive.org/details/fragmenoscien02tyndrich |volume=2 |edition=6th |location=New York |publisher=] |oclc=726998155 }} | |||
| * {{cite book |last1=Voet |first1=Donald |author-link1=Donald Voet |last2=Voet |first2=Judith G. |author-link2=Judith G. Voet |year=2004 |title=Biochemistry |volume=1 |edition=3rd |location=New York |publisher=John Wiley & Sons |isbn=978-0-471-19350-0}} | |||
| * {{cite book |last=Yarus |first=Michael |author-link=Michael Yarus|year=2010 |title=Life from an RNA World: The Ancestor Within |location=Cambridge, Massachusetts |publisher=] |isbn=978-0-674-05075-4 |page=287}} | |||
| {{Refend}} | |||
| == External links == | |||
| {{Library resources box}} | |||
| * – Adam Mann (]; 14 April 2021) | |||
| * {{Webarchive|url=https://web.archive.org/web/20230408001731/https://exploringorigins.org/ |date=8 April 2023 }} a virtual exhibit at the ] | |||
| * – Marcia Malory (Earth Facts; 2015) | |||
| * – ] et al. (]; 2004) | |||
| * – Essay by ] (1996) | |||
| ==External links== | |||
| * Video by ] | |||
| * | |||
| * {{PDFlink||192 KB}} | |||
| * | |||
| * | |||
| * by ] <small>(web archive version as original page no longer accessible)</small> | |||
| * | |||
| * | |||
| * | |||
| * | |||
| * | |||
| * –an article in Scientific American. March 28, 2007 | |||
| * | |||
| * | |||
| {{Origin of life}} | {{Origin of life}} | ||
| {{Nature}} | |||
| {{Branches of biology}} | |||
| {{Molecules detected in outer space}} | |||
| {{Authority control}} | |||
| ] | |||
| ] | |||
| ] | |||
| ] | ] | ||
| ] | ] | ||
| ] | |||
| ] | |||
| {{Link FA|tr}} | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 00:15, 6 January 2025
Life arising from non-living matter "Origin of life" redirects here. For non-scientific views on the origins of life, see Creation myth.

Abiogenesis is the natural process by which life arises from non-living matter, such as simple organic compounds. The prevailing scientific hypothesis is that the transition from non-living to living entities on Earth was not a single event, but a process of increasing complexity involving the formation of a habitable planet, the prebiotic synthesis of organic molecules, molecular self-replication, self-assembly, autocatalysis, and the emergence of cell membranes. The transition from non-life to life has never been observed experimentally, but many proposals have been made for different stages of the process.
The study of abiogenesis aims to determine how pre-life chemical reactions gave rise to life under conditions strikingly different from those on Earth today. It primarily uses tools from biology and chemistry, with more recent approaches attempting a synthesis of many sciences. Life functions through the specialized chemistry of carbon and water, and builds largely upon four key families of chemicals: lipids for cell membranes, carbohydrates such as sugars, amino acids for protein metabolism, and nucleic acid DNA and RNA for the mechanisms of heredity. Any successful theory of abiogenesis must explain the origins and interactions of these classes of molecules.
Many approaches to abiogenesis investigate how self-replicating molecules, or their components, came into existence. Researchers generally think that current life descends from an RNA world, although other self-replicating and self-catalyzing molecules may have preceded RNA. Other approaches ("metabolism-first" hypotheses) focus on understanding how catalysis in chemical systems on the early Earth might have provided the precursor molecules necessary for self-replication. The classic 1952 Miller–Urey experiment demonstrated that most amino acids, the chemical constituents of proteins, can be synthesized from inorganic compounds under conditions intended to replicate those of the early Earth. External sources of energy may have triggered these reactions, including lightning, radiation, atmospheric entries of micro-meteorites, and implosion of bubbles in sea and ocean waves.
While the last universal common ancestor of all modern organisms (LUCA) is thought to have been quite different from the origin of life, investigations into LUCA can guide research into early universal characteristics. A genomics approach has sought to characterize LUCA by identifying the genes shared by Archaea and Bacteria, members of the two major branches of life (with Eukaryotes included in the archaean branch in the two-domain system). It appears there are 60 proteins common to all life and 355 prokaryotic genes that trace to LUCA; their functions imply that the LUCA was anaerobic with the Wood–Ljungdahl pathway, deriving energy by chemiosmosis, and maintaining its hereditary material with DNA, the genetic code, and ribosomes. Although the LUCA lived over 4 billion years ago (4 Gya), researchers believe it was far from the first form of life. Earlier cells might have had a leaky membrane and been powered by a naturally occurring proton gradient near a deep-sea white smoker hydrothermal vent.
Earth remains the only place in the universe known to harbor life. Geochemical and fossil evidence from the Earth informs most studies of abiogenesis. The Earth was formed at 4.54 Gya, and the earliest evidence of life on Earth dates from at least 3.8 Gya from Western Australia. Some studies have suggested that fossil micro-organisms may have lived within hydrothermal vent precipitates dated 3.77 to 4.28 Gya from Quebec, soon after ocean formation 4.4 Gya during the Hadean.
Overview
Further information: Astrobiology
Life consists of reproduction with (heritable) variations. NASA defines life as "a self-sustaining chemical system capable of Darwinian evolution." Such a system is complex; the last universal common ancestor (LUCA), presumably a single-celled organism which lived some 4 billion years ago, already had hundreds of genes encoded in the DNA genetic code that is universal today. That in turn implies a suite of cellular machinery including messenger RNA, transfer RNA, and ribosomes to translate the code into proteins. Those proteins included enzymes to operate its anaerobic respiration via the Wood–Ljungdahl metabolic pathway, and a DNA polymerase to replicate its genetic material.
The challenge for abiogenesis (origin of life) researchers is to explain how such a complex and tightly interlinked system could develop by evolutionary steps, as at first sight all its parts are necessary to enable it to function. For example, a cell, whether the LUCA or in a modern organism, copies its DNA with the DNA polymerase enzyme, which is in turn produced by translating the DNA polymerase gene in the DNA. Neither the enzyme nor the DNA can be produced without the other. The evolutionary process could have involved molecular self-replication, self-assembly such as of cell membranes, and autocatalysis via RNA ribozymes. Nonetheless, the transition of non-life to life has never been observed experimentally, nor has there been a satisfactory chemical explanation.
The preconditions to the development of a living cell like the LUCA are clear enough, though disputed in their details: a habitable world is formed with a supply of minerals and liquid water. Prebiotic synthesis creates a range of simple organic compounds, which are assembled into polymers such as proteins and RNA. On the other side, the process after the LUCA is readily understood: biological evolution caused the development of a wide range of species with varied forms and biochemical capabilities. However, the derivation of living things such as LUCA from simple components is far from understood.
Although Earth remains the only place where life is known, the science of astrobiology seeks evidence of life on other planets. The 2015 NASA strategy on the origin of life aimed to solve the puzzle by identifying interactions, intermediary structures and functions, energy sources, and environmental factors that contributed to the diversity, selection, and replication of evolvable macromolecular systems, and mapping the chemical landscape of potential primordial informational polymers. The advent of polymers that could replicate, store genetic information, and exhibit properties subject to selection was, it suggested, most likely a critical step in the emergence of prebiotic chemical evolution. Those polymers derived, in turn, from simple organic compounds such as nucleobases, amino acids, and sugars that could have been formed by reactions in the environment. A successful theory of the origin of life must explain how all these chemicals came into being.
Pre-1960s conceptual history
Main article: History of research into the origin of life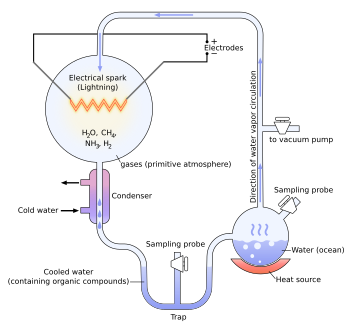
Spontaneous generation
Main article: Spontaneous generationOne ancient view of the origin of life, from Aristotle until the 19th century, is of spontaneous generation. This theory held that "lower" animals such as insects were generated by decaying organic substances, and that life arose by chance. This was questioned from the 17th century, in works like Thomas Browne's Pseudodoxia Epidemica. In 1665, Robert Hooke published the first drawings of a microorganism. In 1676, Antonie van Leeuwenhoek drew and described microorganisms, probably protozoa and bacteria. Van Leeuwenhoek disagreed with spontaneous generation, and by the 1680s convinced himself, using experiments ranging from sealed and open meat incubation and the close study of insect reproduction, that the theory was incorrect. In 1668 Francesco Redi showed that no maggots appeared in meat when flies were prevented from laying eggs. By the middle of the 19th century, spontaneous generation was considered disproven.
Panspermia
Main article: PanspermiaAnother ancient idea dating back to Anaxagoras in the 5th century BC is panspermia, the idea that life originated elsewhere in the universe and came to Earth. The modern version of panspermia holds that life may have been distributed to Earth by meteoroids, asteroids, comets and planetoids. It does not attempt to explain how life originated in itself, but shifts the origin of life on Earth to another heavenly body. The advantage is that life is not required to have formed on each planet it occurs on, but rather in a more limited set of locations, or even a single location, and then spread about the galaxy to other star systems via cometary or meteorite impact. Panspermia did not get much scientific support because it was largely used to deflect the need of an answer instead of explaining observable phenomena. Although the interest in panspermia grew when the study of meteorites found traces of organic materials in them, it is currently accepted that life started locally on Earth.
"A warm little pond": primordial soup
Main article: Primordial soupThe idea that life originated from non-living matter in slow stages appeared in Herbert Spencer's 1864–1867 book Principles of Biology, and in William Turner Thiselton-Dyer's 1879 paper "On spontaneous generation and evolution". On 1 February 1871 Charles Darwin wrote about these publications to Joseph Hooker, and set out his own speculation, suggesting that the original spark of life may have begun in a "warm little pond, with all sorts of ammonia and phosphoric salts, light, heat, electricity, &c., present, that a proteine compound was chemically formed ready to undergo still more complex changes." Darwin went on to explain that "at the present day such matter would be instantly devoured or absorbed, which would not have been the case before living creatures were formed."
Alexander Oparin in 1924 and J. B. S. Haldane in 1929 proposed that the first molecules constituting the earliest cells slowly self-organized from a primordial soup, and this theory is called the Oparin–Haldane hypothesis. Haldane suggested that the Earth's prebiotic oceans consisted of a "hot dilute soup" in which organic compounds could have formed. J. D. Bernal showed that such mechanisms could form most of the necessary molecules for life from inorganic precursors. In 1967, he suggested three "stages": the origin of biological monomers; the origin of biological polymers; and the evolution from molecules to cells.
Miller–Urey experiment
Main article: Miller–Urey experimentIn 1952, Stanley Miller and Harold Urey carried out a chemical experiment to demonstrate how organic molecules could have formed spontaneously from inorganic precursors under prebiotic conditions like those posited by the Oparin–Haldane hypothesis. It used a highly reducing (lacking oxygen) mixture of gases—methane, ammonia, and hydrogen, as well as water vapor—to form simple organic monomers such as amino acids. Bernal said of the Miller–Urey experiment that "it is not enough to explain the formation of such molecules, what is necessary, is a physical-chemical explanation of the origins of these molecules that suggests the presence of suitable sources and sinks for free energy." However, current scientific consensus describes the primitive atmosphere as weakly reducing or neutral, diminishing the amount and variety of amino acids that could be produced. The addition of iron and carbonate minerals, present in early oceans, however, produces a diverse array of amino acids. Later work has focused on two other potential reducing environments: outer space and deep-sea hydrothermal vents.
Producing a habitable Earth
| Origin of life timeline | ||||||||||||||||||||||||
| −13 —–−12 —–−11 —–−10 —–−9 —–−8 —–−7 —–−6 —–−5 —–−4 —–−3 —–−2 —–−1 —–0 — | Dark AgesReionizationChemical elementsOrganic compoundsWater on EarthSingle-celled lifePhotosynthesisMulticellular life |
i f e | ||||||||||||||||||||||
| (billion years ago) | ||||||||||||||||||||||||
Evolutionary history
Early universe with first stars
See also: Chronology of the universeSoon after the Big Bang, which occurred roughly 14 Gya, the only chemical elements present in the universe were hydrogen, helium, and lithium, the three lightest atoms in the periodic table. These elements gradually accreted and began orbiting in disks of gas and dust. Gravitational accretion of material at the hot and dense centers of these protoplanetary disks formed stars by the fusion of hydrogen. Early stars were massive and short-lived, producing all the heavier elements through stellar nucleosynthesis. Element formation through stellar nucleosynthesis proceeds to its most stable element Iron-56. Heavier elements were formed during supernovae at the end of a stars lifecycle. Carbon, currently the fourth most abundant chemical element in the universe (after hydrogen, helium, and oxygen), was formed mainly in white dwarf stars, particularly those bigger than twice the mass of the sun. As these stars reached the end of their lifecycles, they ejected these heavier elements, among them carbon and oxygen, throughout the universe. These heavier elements allowed for the formation of new objects, including rocky planets and other bodies. According to the nebular hypothesis, the formation and evolution of the Solar System began 4.6 Gya with the gravitational collapse of a small part of a giant molecular cloud. Most of the collapsing mass collected in the center, forming the Sun, while the rest flattened into a protoplanetary disk out of which the planets, moons, asteroids, and other small Solar System bodies formed.
Emergence of Earth
See also: Geological history of Earth, Circumstellar habitable zone, and Prebiotic atmosphereThe age of the Earth is 4.54 Gya as found by radiometric dating of calcium-aluminium-rich inclusions in carbonaceous chrondrite meteorites, the oldest material in the Solar System. Earth, during the Hadean eon (from its formation until 4.031 Gya,) was at first inhospitable to any living organisms. During its formation, the Earth lost a significant part of its initial mass, and consequentially lacked the gravity to hold molecular hydrogen and the bulk of the original inert gases. Soon after initial accretion of Earth at 4.48 Ga, its collision with Theia, a hypothesised impactor, is thought to have created the ejected debris that would eventually form the Moon. This impact would have removed the Earth's primary atmosphere, leaving behind clouds of viscous silicates and carbon dioxide. This unstable atmosphere was short-lived and condensed shortly after to form the bulk silicate Earth, leaving behind an atmosphere largely consisting of water vapor, nitrogen, and carbon dioxide, with smaller amounts of carbon monoxide, hydrogen, and sulfur compounds. The solution of carbon dioxide in water is thought to have made the seas slightly acidic, with a pH of about 5.5.
Condensation to form liquid oceans is theorised to have occurred as early as the Moon-forming impact. This scenario has found support from the dating of 4.404 Gya zircon crystals with high δO values from metamorphosed quartzite of Mount Narryer in Western Australia. The Hadean atmosphere has been characterized as a "gigantic, productive outdoor chemical laboratory," similar to volcanic gases today which still support some abiotic chemistry. Despite the likely increased volcanism from early plate tectonics, the Earth may have been a predominantly water world between 4.4 and 4.3 Gya. It is debated whether or not crust was exposed above this ocean due to uncertainties of what early plate tectonics looked like. For early life to have developed, it is generally thought that a land setting is required, so this question is essential to determining when in Earth's history life evolved. Immediately after the Moon-forming impact, Earth likely had little if any continental crust, a turbulent atmosphere, and a hydrosphere subject to intense ultraviolet light from a T Tauri stage Sun. It was also affected by cosmic radiation, and continued asteroid and comet impacts. Despite all this, niche environments likely existed conducive to life on Earth in the Late-Hadean to Early-Archaean.
The Late Heavy Bombardment hypothesis posits that a period of intense impact occurred at 4.1 to 3.8 Gya during the Hadean and early Archean eons. Originally it was thought that the Late Heavy Bombardment was a single cataclysmic impact event occurring at 3.9 Gya; this would have had the potential to sterilise all life on Earth by volatilising liquid oceans and blocking the Sun needed for photosynthesising primary producers, pushing back the earliest possible emergence of life to after the Late Heavy Bombardment. However, more recent research questioned both the intensity of the Late Heavy Bombardment as well as its potential for sterilisation. Uncertainties as to whether Late Heavy Bombardment was one giant impact or a period of greater impact rates greatly changed the implication of its destructive power. The 3.9 Ga date arose from dating of Apollo mission sample returns collected mostly near the Imbrium Basin, biasing the age of recorded impacts. Impact modelling of the lunar surface reveals that rather than a cataclysmic event at 3.9 Ga, multiple small-scale, short-lived periods of bombardment likely occurred. Terrestrial data backs this idea by showing multiple periods of ejecta in the rock record both before and after the 3.9 Ga marker, suggesting that the early Earth was subject to continuous impacts that would not have had as great an impact on extinction as previously thought. If the Late Heavy Bombardment was not a single cataclysmic event, the emergence of life could have taken place far before 3.9 Ga.
If life evolved in the ocean at depths of more than ten meters, it would have been shielded both from late impacts and the then high levels of ultraviolet radiation from the sun. Geothermically heated oceanic crust could have yielded far more organic compounds through deep hydrothermal vents than the Miller–Urey experiments indicated. The available energy is maximized at 100–150 °C, the temperatures at which hyperthermophilic bacteria and thermoacidophilic archaea live.
Earliest evidence of life
Main article: Earliest known life formsThe exact timing at which life emerged on Earth is unknown. Minimum age estimates are based on evidence from the geologic rock record. The earliest physical evidence of life so far found consists of microbialites in the Nuvvuagittuq Greenstone Belt of Northern Quebec, in banded iron formation rocks at least 3.77 and possibly as old as 4.32 Gya. The micro-organisms could have lived within hydrothermal vent precipitates, soon after the 4.4 Gya formation of oceans during the Hadean. The microbes resembled modern hydrothermal vent bacteria, supporting the view that abiogenesis began in such an environment.
Biogenic graphite has been found in 3.7 Gya metasedimentary rocks from southwestern Greenland and in microbial mat fossils from 3.49 Gya cherts in the Pilbara region of Western Australia. Evidence of early life in rocks from Akilia Island, near the Isua supracrustal belt in southwestern Greenland, dating to 3.7 Gya, have shown biogenic carbon isotopes. In other parts of the Isua supracrustal belt, graphite inclusions trapped within garnet crystals are connected to the other elements of life: oxygen, nitrogen, and possibly phosphorus in the form of phosphate, providing further evidence for life 3.7 Gya. In the Pilbara region of Western Australia, compelling evidence of early life was found in pyrite-bearing sandstone in a fossilized beach, with rounded tubular cells that oxidized sulfur by photosynthesis in the absence of oxygen. Carbon isotope ratios on graphite inclusions from the Jack Hills zircons suggest that life could have existed on Earth from 4.1 Gya.
The Pilbara region of Western Australia contains the Dresser Formation with rocks 3.48 Gya, including layered structures called stromatolites. Their modern counterparts are created by photosynthetic micro-organisms including cyanobacteria. These lie within undeformed hydrothermal-sedimentary strata; their texture indicates a biogenic origin. Parts of the Dresser formation preserve hot springs on land, but other regions seem to have been shallow seas. A molecular clock analysis suggests the LUCA emerged prior to 3.9 Gya.
-
 Fossilized stromatolites in the Siyeh Formation, Glacier National Park, dated 3.5 Gya, placing them among the earliest life-forms
Fossilized stromatolites in the Siyeh Formation, Glacier National Park, dated 3.5 Gya, placing them among the earliest life-forms
-
 Modern stromatolites in Shark Bay, created by photosynthetic cyanobacteria
Modern stromatolites in Shark Bay, created by photosynthetic cyanobacteria
Producing molecules: prebiotic synthesis
Further information: NucleosynthesisAll chemical elements derive from stellar nucleosynthesis except for hydrogen and some helium and lithium. Basic chemical ingredients of life – the carbon-hydrogen molecule (CH), the carbon-hydrogen positive ion (CH+) and the carbon ion (C+) – can be produced by ultraviolet light from stars. Complex molecules, including organic molecules, form naturally both in space and on planets. Organic molecules on the early Earth could have had either terrestrial origins, with organic molecule synthesis driven by impact shocks or by other energy sources, such as ultraviolet light, redox coupling, or electrical discharges; or extraterrestrial origins (pseudo-panspermia), with organic molecules formed in interstellar dust clouds raining down on to the planet.
Observed extraterrestrial organic molecules
See also: List of interstellar and circumstellar molecules and Pseudo-panspermiaAn organic compound is a chemical whose molecules contain carbon. Carbon is abundant in the Sun, stars, comets, and in the atmospheres of most planets of the Solar System. Organic compounds are relatively common in space, formed by "factories of complex molecular synthesis" which occur in molecular clouds and circumstellar envelopes, and chemically evolve after reactions are initiated mostly by ionizing radiation. Purine and pyrimidine nucleobases including guanine, adenine, cytosine, uracil, and thymine have been found in meteorites. These could have provided the materials for DNA and RNA to form on the early Earth. The amino acid glycine was found in material ejected from comet Wild 2; it had earlier been detected in meteorites. Comets are encrusted with dark material, thought to be a tar-like organic substance formed from simple carbon compounds under ionizing radiation. A rain of material from comets could have brought such complex organic molecules to Earth. It is estimated that during the Late Heavy Bombardment, meteorites may have delivered up to five million tons of organic prebiotic elements to Earth per year. Currently 40,000 tons of cosmic dust falls to Earth each year.
Polycyclic aromatic hydrocarbons

Green areas show regions where radiation from hot stars collided with large molecules and small dust grains called "polycyclic aromatic hydrocarbons" (PAHs), causing them to fluoresce. Spitzer Space Telescope, 2018.
Polycyclic aromatic hydrocarbons (PAH) are the most common and abundant polyatomic molecules in the observable universe, and are a major store of carbon. They seem to have formed shortly after the Big Bang, and are associated with new stars and exoplanets. They are a likely constituent of Earth's primordial sea. PAHs have been detected in nebulae, and in the interstellar medium, in comets, and in meteorites.
A star, HH 46-IR, resembling the sun early in its life, is surrounded by a disk of material which contains molecules including cyanide compounds, hydrocarbons, and carbon monoxide. PAHs in the interstellar medium can be transformed through hydrogenation, oxygenation, and hydroxylation to more complex organic compounds used in living cells.
Nucleobases and nucleotides
Further information: Nucleobase and NucleotideOrganic compounds introduced on Earth by interstellar dust particles can help to form complex molecules, thanks to their peculiar surface-catalytic activities. The RNA component uracil and related molecules, including xanthine, in the Murchison meteorite were likely formed extraterrestrially, as suggested by studies of C/C isotopic ratios. NASA studies of meteorites suggest that all four DNA nucleobases (adenine, guanine and related organic molecules) have been formed in outer space. The cosmic dust permeating the universe contains complex organics ("amorphous organic solids with a mixed aromatic–aliphatic structure") that could be created rapidly by stars. Glycolaldehyde, a sugar molecule and RNA precursor, has been detected in regions of space including around protostars and on meteorites.
Laboratory synthesis
As early as the 1860s, experiments demonstrated that biologically relevant molecules can be produced from interaction of simple carbon sources with abundant inorganic catalysts. The spontaneous formation of complex polymers from abiotically generated monomers under the conditions posited by the "soup" theory is not straightforward. Besides the necessary basic organic monomers, compounds that would have prohibited the formation of polymers were also formed in high concentration during the Miller–Urey and Joan Oró experiments. Biology uses essentially 20 amino acids for its coded protein enzymes, representing a very small subset of the structurally possible products. Since life tends to use whatever is available, an explanation is needed for why the set used is so small. Formamide is attractive as a medium that potentially provided a source of amino acid derivatives from simple aldehyde and nitrile feedstocks.
Sugars

Alexander Butlerov showed in 1861 that the formose reaction created sugars including tetroses, pentoses, and hexoses when formaldehyde is heated under basic conditions with divalent metal ions like calcium. R. Breslow proposed that the reaction was autocatalytic in 1959.
Nucleobases
Nucleobases, such as guanine and adenine, can be synthesized from simple carbon and nitrogen sources, such as hydrogen cyanide (HCN) and ammonia. Formamide produces all four ribonucleotides when warmed with terrestrial minerals. Formamide is ubiquitous in the Universe, produced by the reaction of water and HCN. It can be concentrated by the evaporation of water. HCN is poisonous only to aerobic organisms (eukaryotes and aerobic bacteria), which did not yet exist. It can play roles in other chemical processes such as the synthesis of the amino acid glycine.
DNA and RNA components including uracil, cytosine and thymine can be synthesized under outer space conditions, using starting chemicals such as pyrimidine found in meteorites. Pyrimidine may have been formed in red giant stars or in interstellar dust and gas clouds. All four RNA-bases may be synthesized from formamide in high-energy density events like extraterrestrial impacts.
Other pathways for synthesizing bases from inorganic materials have been reported. Freezing temperatures are advantageous for the synthesis of purines, due to the concentrating effect for key precursors such as hydrogen cyanide. However, while adenine and guanine require freezing conditions for synthesis, cytosine and uracil may require boiling temperatures. Seven amino acids and eleven types of nucleobases formed in ice when ammonia and cyanide were left in a freezer for 25 years. S-triazines (alternative nucleobases), pyrimidines including cytosine and uracil, and adenine can be synthesized by subjecting a urea solution to freeze-thaw cycles under a reductive atmosphere, with spark discharges as an energy source. The explanation given for the unusual speed of these reactions at such a low temperature is eutectic freezing, which crowds impurities in microscopic pockets of liquid within the ice, causing the molecules to collide more often.
Peptides
Prebiotic peptide synthesis is proposed to have occurred through a number of possible routes. Some center on high temperature/concentration conditions in which condensation becomes energetically favorable, while others focus on the availability of plausible prebiotic condensing agents.
Experimental evidence for the formation of peptides in uniquely concentrated environments is bolstered by work suggesting that wet-dry cycles and the presence of specific salts can greatly increase spontaneous condensation of glycine into poly-glycine chains. Other work suggests that while mineral surfaces, such as those of pyrite, calcite, and rutile catalyze peptide condensation, they also catalyze their hydrolysis. The authors suggest that additional chemical activation or coupling would be necessary to produce peptides at sufficient concentrations. Thus, mineral surface catalysis, while important, is not sufficient alone for peptide synthesis.
Many prebiotically plausible condensing/activating agents have been identified, including the following: cyanamide, dicyanamide, dicyandiamide, diaminomaleonitrile, urea, trimetaphosphate, NaCl, CuCl2, (Ni,Fe)S, CO, carbonyl sulfide (COS), carbon disulfide (CS2), SO2, and diammonium phosphate (DAP).
An experiment reported in 2024 used a sapphire substrate with a web of thin cracks under a heat flow, similar to the environment of deep-ocean vents, as a mechanism to separate and concentrate prebiotically relevant building blocks from a dilute mixture, purifying their concentration by up to three orders of magnitude. The authors propose this as a plausible model for the origin of complex biopolymers. This presents another physical process that allows for concentrated peptide precursors to combine in the right conditions. A similar role of increasing amino acid concentration has been suggested for clays as well.
While all of these scenarios involve the condensation of amino acids, the prebiotic synthesis of peptides from simpler molecules such as CO, NH3 and C, skipping the step of amino acid formation, is very efficient.
Producing suitable vesicles
Further information: Gard model, Self-organization § Biology, and Cellularization
The largest unanswered question in evolution is how simple protocells first arose and differed in reproductive contribution to the following generation, thus initiating the evolution of life. The lipid world theory postulates that the first self-replicating object was lipid-like. Phospholipids form lipid bilayers in water while under agitation—the same structure as in cell membranes. These molecules were not present on early Earth, but other amphiphilic long-chain molecules also form membranes. These bodies may expand by insertion of additional lipids, and may spontaneously split into two offspring of similar size and composition. Lipid bodies may have provided sheltering envelopes for information storage, allowing the evolution and preservation of polymers like RNA that store information. Only one or two types of amphiphiles have been studied which might have led to the development of vesicles. There is an enormous number of possible arrangements of lipid bilayer membranes, and those with the best reproductive characteristics would have converged toward a hypercycle reaction, a positive feedback composed of two mutual catalysts represented by a membrane site and a specific compound trapped in the vesicle. Such site/compound pairs are transmissible to the daughter vesicles leading to the emergence of distinct lineages of vesicles, which would have allowed natural selection.
A protocell is a self-organized, self-ordered, spherical collection of lipids proposed as a stepping-stone to the origin of life. A functional protocell has (as of 2014) not yet been achieved in a laboratory setting. Self-assembled vesicles are essential components of primitive cells. The theory of classical irreversible thermodynamics treats self-assembly under a generalized chemical potential within the framework of dissipative systems. The second law of thermodynamics requires that overall entropy increases, yet life is distinguished by its great degree of organization. Therefore, a boundary is needed to separate ordered life processes from chaotic non-living matter.
Irene Chen and Jack W. Szostak suggest that elementary protocells can give rise to cellular behaviors including primitive forms of differential reproduction, competition, and energy storage. Competition for membrane molecules would favor stabilized membranes, suggesting a selective advantage for the evolution of cross-linked fatty acids and even the phospholipids of today. Such micro-encapsulation would allow for metabolism within the membrane and the exchange of small molecules, while retaining large biomolecules inside. Such a membrane is needed for a cell to create its own electrochemical gradient to store energy by pumping ions across the membrane. Fatty acid vesicles in conditions relevant to alkaline hydrothermal vents can be stabilized by isoprenoids which are synthesized by the formose reaction; the advantages and disadvantages of isoprenoids incorporated within the lipid bilayer in different microenvironments might have led to the divergence of the membranes of archaea and bacteria.
Laboratory experiments have shown that vesicles can undergo an evolutionary process under pressure cycling conditions. Simulating the systemic environment in tectonic fault zones within the Earth's crust, pressure cycling leads to the periodic formation of vesicles. Under the same conditions, random peptide chains are being formed, which are being continuously selected for their ability to integrate into the vesicle membrane. A further selection of the vesicles for their stability potentially leads to the development of functional peptide structures, associated with an increase in the survival rate of the vesicles.
Producing biology
Energy and entropy
Further information: EntropyLife requires a loss of entropy, or disorder, as molecules organize themselves into living matter. At the same time, the emergence of life is associated with the formation of structures beyond a certain threshold of complexity. The emergence of life with increasing order and complexity does not contradict the second law of thermodynamics, which states that overall entropy never decreases, since a living organism creates order in some places (e.g. its living body) at the expense of an increase of entropy elsewhere (e.g. heat and waste production).
Multiple sources of energy were available for chemical reactions on the early Earth. Heat from geothermal processes is a standard energy source for chemistry. Other examples include sunlight, lightning, atmospheric entries of micro-meteorites, and implosion of bubbles in sea and ocean waves. This has been confirmed by experiments and simulations. Unfavorable reactions can be driven by highly favorable ones, as in the case of iron-sulfur chemistry. For example, this was probably important for carbon fixation. Carbon fixation by reaction of CO2 with H2S via iron-sulfur chemistry is favorable, and occurs at neutral pH and 100 °C. Iron-sulfur surfaces, which are abundant near hydrothermal vents, can drive the production of small amounts of amino acids and other biomolecules.
Chemiosmosis
Further information: Chemiosmosis
In 1961, Peter Mitchell proposed chemiosmosis as a cell's primary system of energy conversion. The mechanism, now ubiquitous in living cells, powers energy conversion in micro-organisms and in the mitochondria of eukaryotes, making it a likely candidate for early life. Mitochondria produce adenosine triphosphate (ATP), the energy currency of the cell used to drive cellular processes such as chemical syntheses. The mechanism of ATP synthesis involves a closed membrane in which the ATP synthase enzyme is embedded. The energy required to release strongly bound ATP has its origin in protons that move across the membrane. In modern cells, those proton movements are caused by the pumping of ions across the membrane, maintaining an electrochemical gradient. In the first organisms, the gradient could have been provided by the difference in chemical composition between the flow from a hydrothermal vent and the surrounding seawater, or perhaps meteoric quinones that were conducive to the development of chemiosmotic energy across lipid membranes if at a terrestrial origin.

PAH world hypothesis
Main article: PAH world hypothesisThe PAH world hypothesis posits polycyclic aromatic hydrocarbons as precursors to the RNA world.
This section is an excerpt from PAH world hypothesis. The PAH world hypothesis is a speculative hypothesis that proposes that polycyclic aromatic hydrocarbons (PAHs), known to be abundant in the universe, including in comets, and assumed to be abundant in the primordial soup of the early Earth, played a major role in the origin of life by mediating the synthesis of RNA molecules, leading into the RNA world. However, as yet, the hypothesis is untested.The RNA world
Main article: RNA worldThe RNA world hypothesis describes an early Earth with self-replicating and catalytic RNA but no DNA or proteins. Many researchers concur that an RNA world must have preceded the DNA-based life that now dominates. However, RNA-based life may not have been the first to exist. Another model echoes Darwin's "warm little pond" with cycles of wetting and drying.
RNA is central to the translation process. Small RNAs can catalyze all the chemical groups and information transfers required for life. RNA both expresses and maintains genetic information in modern organisms; and the chemical components of RNA are easily synthesized under the conditions that approximated the early Earth, which were very different from those that prevail today. The structure of the ribosome has been called the "smoking gun", with a central core of RNA and no amino acid side chains within 18 Å of the active site that catalyzes peptide bond formation.
The concept of the RNA world was proposed in 1962 by Alexander Rich, and the term was coined by Walter Gilbert in 1986. There were initial difficulties in the explanation of the abiotic synthesis of the nucleotides cytosine and uracil. Subsequent research has shown possible routes of synthesis; for example, formamide produces all four ribonucleotides and other biological molecules when warmed in the presence of various terrestrial minerals.

RNA replicase can function as both code and catalyst for further RNA replication, i.e. it can be autocatalytic. Jack Szostak has shown that certain catalytic RNAs can join smaller RNA sequences together, creating the potential for self-replication. The RNA replication systems, which include two ribozymes that catalyze each other's synthesis, showed a doubling time of the product of about one hour, and were subject to natural selection under the experimental conditions. If such conditions were present on early Earth, then natural selection would favor the proliferation of such autocatalytic sets, to which further functionalities could be added. Self-assembly of RNA may occur spontaneously in hydrothermal vents. A preliminary form of tRNA could have assembled into such a replicator molecule.
Possible precursors to protein synthesis include the synthesis of short peptide cofactors or the self-catalysing duplication of RNA. It is likely that the ancestral ribosome was composed entirely of RNA, although some roles have since been taken over by proteins. Major remaining questions on this topic include identifying the selective force for the evolution of the ribosome and determining how the genetic code arose.
Eugene Koonin has argued that "no compelling scenarios currently exist for the origin of replication and translation, the key processes that together comprise the core of biological systems and the apparent pre-requisite of biological evolution. The RNA World concept might offer the best chance for the resolution of this conundrum but so far cannot adequately account for the emergence of an efficient RNA replicase or the translation system."
From RNA to directed protein synthesis
In line with the RNA world hypothesis, much of modern biology's templated protein biosynthesis is done by RNA molecules—namely tRNAs and the ribosome (consisting of both protein and rRNA components). The most central reaction of peptide bond synthesis is understood to be carried out by base catalysis by the 23S rRNA domain V. Experimental evidence has demonstrated successful di- and tripeptide synthesis with a system consisting of only aminoacyl phosphate adaptors and RNA guides, which could be a possible stepping stone between an RNA world and modern protein synthesis. Aminoacylation ribozymes that can charge tRNAs with their cognate amino acids have also been selected in in vitro experimentation. The authors also extensively mapped fitness landscapes within their selection to find that chance emergence of active sequences was more important that sequence optimization.
Early functional peptides
The first proteins would have had to arise without a fully-fledged system of protein biosynthesis. As discussed above, numerous mechanisms for the prebiotic synthesis of polypeptides exist. However, these random sequence peptides would not have likely had biological function. Thus, significant study has gone into exploring how early functional proteins could have arisen from random sequences. First, some evidence on hydrolysis rates shows that abiotically plausible peptides likely contained significant "nearest-neighbor" biases. This could have had some effect on early protein sequence diversity. In other work by Anthony Keefe and Jack Szostak, mRNA display selection on a library of 6*10 80-mers was used to search for sequences with ATP binding activity. They concluded that approximately 1 in 10 random sequences had ATP binding function. While this is a single example of functional frequency in the random sequence space, the methodology can serve as a powerful simulation tool for understanding early protein evolution.
Phylogeny and LUCA
Further information: Last universal common ancestorStarting with the work of Carl Woese from 1977, genomics studies have placed the last universal common ancestor (LUCA) of all modern life-forms between Bacteria and a clade formed by Archaea and Eukaryota in the phylogenetic tree of life. It lived over 4 Gya. A minority of studies have placed the LUCA in Bacteria, proposing that Archaea and Eukaryota are evolutionarily derived from within Eubacteria; Thomas Cavalier-Smith suggested in 2006 that the phenotypically diverse bacterial phylum Chloroflexota contained the LUCA.
-
 Phylogenetic tree showing the last universal common ancestor (LUCA) at the root. The major clades are the Bacteria on one hand, and the Archaea and Eukaryota on the other.
Phylogenetic tree showing the last universal common ancestor (LUCA) at the root. The major clades are the Bacteria on one hand, and the Archaea and Eukaryota on the other.
In 2016, a set of 355 genes likely present in the LUCA was identified. A total of 6.1 million prokaryotic genes from Bacteria and Archaea were sequenced, identifying 355 protein clusters from among 286,514 protein clusters that were probably common to the LUCA. The results suggest that the LUCA was anaerobic with a Wood–Ljungdahl (reductive Acetyl-CoA) pathway, nitrogen- and carbon-fixing, thermophilic. Its cofactors suggest dependence upon an environment rich in hydrogen, carbon dioxide, iron, and transition metals. Its genetic material was probably DNA, requiring the 4-nucleotide genetic code, messenger RNA, transfer RNA, and ribosomes to translate the code into proteins such as enzymes. LUCA likely inhabited an anaerobic hydrothermal vent setting in a geochemically active environment. It was evidently already a complex organism, and must have had precursors; it was not the first living thing. The physiology of LUCA has been in dispute. Previous research identified 60 proteins common to all life.
-
 LUCA systems and environment included the Wood–Ljungdahl pathway.
LUCA systems and environment included the Wood–Ljungdahl pathway.
Leslie Orgel argued that early translation machinery for the genetic code would be susceptible to error catastrophe. Geoffrey Hoffmann however showed that such machinery can be stable in function against "Orgel's paradox". Metabolic reactions that have also been inferred in LUCA are the incomplete reverse Krebs cycle, gluconeogenesis, the pentose phosphate pathway, glycolysis, reductive amination, and transamination.
Suitable geological environments
Further information: Alternative abiogenesis scenariosA variety of geologic and environmental settings have been proposed for an origin of life. These theories are often in competition with one another as there are many differing views of prebiotic compound availability, geophysical setting, and early life characteristics. The first organism on Earth likely looked different from LUCA. Between the first appearance of life and where all modern phylogenies began branching, an unknown amount of time passed, with unknown gene transfers, extinctions, and evolutionary adaptation to various environmental niches. One major shift is believed to be from the RNA world to an RNA-DNA-protein world. Modern phylogenies provide more pertinent genetic evidence about LUCA than about its precursors.
The most popular hypotheses for settings for the origin of life are deep sea hydrothermal vents and surface bodies of water. Surface waters can be classified into hot springs, moderate temperature lakes and ponds, and cold settings.
Deep sea hydrothermal vents
Hot fluids
Further information: Hydrothermal vent
Early micro-fossils may have come from a hot world of gases such as methane, ammonia, carbon dioxide, and hydrogen sulfide, toxic to much current life. Analysis of the tree of life places thermophilic and hyperthermophilic bacteria and archaea closest to the root, suggesting that life may have evolved in a hot environment. The deep sea or alkaline hydrothermal vent theory posits that life began at submarine hydrothermal vents. William Martin and Michael Russell have suggested "that life evolved in structured iron monosulphide precipitates in a seepage site hydrothermal mound at a redox, pH, and temperature gradient between sulphide-rich hydrothermal fluid and iron(II)-containing waters of the Hadean ocean floor. The naturally arising, three-dimensional compartmentation observed within fossilized seepage-site metal sulphide precipitates indicates that these inorganic compartments were the precursors of cell walls and membranes found in free-living prokaryotes. The known capability of FeS and NiS to catalyze the synthesis of the acetyl-methylsulphide from carbon monoxide and methylsulphide, constituents of hydrothermal fluid, indicates that pre-biotic syntheses occurred at the inner surfaces of these metal-sulphide-walled compartments".
These form where hydrogen-rich fluids emerge from below the sea floor, as a result of serpentinization of ultra-mafic olivine with seawater and a pH interface with carbon dioxide-rich ocean water. The vents form a sustained chemical energy source derived from redox reactions, in which electron donors (molecular hydrogen) react with electron acceptors (carbon dioxide); see iron–sulfur world theory. These are exothermic reactions.
Chemiosmotic gradient
Further information: Hydrothermal vent and Chemiosmosis § Emergence of chemiosmosis
Russell demonstrated that alkaline vents created an abiogenic proton motive force chemiosmotic gradient, ideal for abiogenesis. Their microscopic compartments "provide a natural means of concentrating organic molecules," composed of iron-sulfur minerals such as mackinawite, endowed these mineral cells with the catalytic properties envisaged by Günter Wächtershäuser. This movement of ions across the membrane depends on a combination of two factors:
- Diffusion force caused by concentration gradient—all particles including ions tend to diffuse from higher concentration to lower.
- Electrostatic force caused by electrical potential gradient—cations like protons H tend to diffuse down the electrical potential, anions in the opposite direction.
These two gradients taken together can be expressed as an electrochemical gradient, providing energy for abiogenic synthesis. The proton motive force can be described as the measure of the potential energy stored as a combination of proton and voltage gradients across a membrane (differences in proton concentration and electrical potential).
The surfaces of mineral particles inside deep-ocean hydrothermal vents have catalytic properties similar to those of enzymes and can create simple organic molecules, such as methanol (CH3OH) and formic, acetic, and pyruvic acids out of the dissolved CO2 in the water, if driven by an applied voltage or by reaction with H2 or H2S.
Starting in 1985, researchers proposed that life arose at hydrothermal vents, that spontaneous chemistry in the Earth's crust driven by rock–water interactions at disequilibrium thermodynamically underpinned life's origin and that the founding lineages of the archaea and bacteria were H2-dependent autotrophs that used CO2 as their terminal acceptor in energy metabolism. In 2016, Martin suggested, based upon this evidence, that the LUCA "may have depended heavily on the geothermal energy of the vent to survive". Pores at deep sea hydrothermal vents are suggested to have been occupied by membrane-bound compartments which promoted biochemical reactions. Metabolic intermediates in the Krebs cycle, gluconeogenesis, amino acid bio-synthetic pathways, glycolysis, the pentose phosphate pathway, and including sugars like ribose, and lipid precursors can occur non-enzymatically at conditions relevant to deep-sea alkaline hydrothermal vents.
If the deep marine hydrothermal setting was the site for the origin of life, then abiogenesis could have happened as early as 4.0-4.2 Gya. If life evolved in the ocean at depths of more than ten meters, it would have been shielded both from impacts and the then high levels of ultraviolet radiation from the sun. The available energy in hydrothermal vents is maximized at 100–150 °C, the temperatures at which hyperthermophilic bacteria and thermoacidophilic archaea live. Arguments against a hydrothermal origin of life state that hyperthermophily was a result of convergent evolution in bacteria and archaea, and that a mesophilic environment would have been more likely. This hypothesis, suggested in 1999 by Galtier, was proposed one year before the discovery of the Lost City Hydrothermal Field, where white-smoker hydrothermal vents average ~45-90 °C. Moderate temperatures and alkaline seawater such as that at Lost City are now the favoured hydrothermal vent setting in contrast to acidic, high temperature (~350 °C) black-smokers.
Arguments against a vent setting
Production of prebiotic organic compounds at hydrothermal vents is estimated to be 1x10 kg yr. While a large amount of key prebiotic compounds, such as methane, are found at vents, they are in far lower concentrations than estimates of a Miller-Urey Experiment environment. In the case of methane, the production rate at vents is around 2-4 orders of magnitude lower than predicted amounts in a Miller-Urey Experiment surface atmosphere.
Other arguments against an oceanic vent setting for the origin of life include the inability to concentrate prebiotic materials due to strong dilution from seawater. This open-system cycles compounds through minerals that make up vents, leaving little residence time to accumulate. All modern cells rely on phosphates and potassium for nucleotide backbone and protein formation respectively, making it likely that the first life forms also shared these functions. These elements were not available in high quantities in the Archaean oceans as both primarily come from the weathering of continental rocks on land, far from vent settings. Submarine hydrothermal vents are not conducive to condensation reactions needed for polymerisation to form macromolecules.
An older argument was that key polymers were encapsulated in vesicles after condensation, which supposedly would not happen in saltwater because of the high concentrations of ions. However, while it is true that salinity inhibits vesicle formation from low-diversity mixtures of fatty acids, vesicle formation from a broader, more realistic mix of fatty-acid and 1-alkanol species is more resilient.
Surface bodies of water
Surface bodies of water provide environments able to dry out and be rewetted. Continued wet-dry cycles allow the concentration of prebiotic compounds and condensation reactions to polymerise macromolecules. Moreover, lake and ponds on land allow for detrital input from the weathering of continental rocks which contain apatite, the most common source of phosphates needed for nucleotide backbones. The amount of exposed continental crust in the Hadean is unknown, but models of early ocean depths and rates of ocean island and continental crust growth make it plausible that there was exposed land. Another line of evidence for a surface start to life is the requirement for UV for organism function. UV is necessary for the formation of the U+C nucleotide base pair by partial hydrolysis and nucleobase loss. Simultaneously, UV can be harmful and sterilising to life, especially for simple early lifeforms with little ability to repair radiation damage. Radiation levels from a young Sun were likely greater, and, with no ozone layer, harmful shortwave UV rays would reach the surface of Earth. For life to begin, a shielded environment with influx from UV-exposed sources is necessary to both benefit and protect from UV. Shielding under ice, liquid water, mineral surfaces (e.g. clay) or regolith is possible in a range of surface water settings. While deep sea vents may have input from raining down of surface exposed materials, the likelihood of concentration is lessened by the ocean's open system.
Hot springs
Most branching phylogenies are thermophilic or hyperthermophilic, making it possible that the Last universal common ancestor (LUCA) and preceding lifeforms were similarly thermophilic. Hot springs are formed from the heating of groundwater by geothermal activity. This intersection allows for influxes of material from deep penetrating waters and from surface runoff that transports eroded continental sediments. Interconnected groundwater systems create a mechanism for distribution of life to wider area.
Mulkidjanian and co-authors argue that marine environments did not provide the ionic balance and composition universally found in cells, or the ions required by essential proteins and ribozymes, especially with respect to high K/Na ratio, Mn, Zn and phosphate concentrations. They argue that the only environments that mimic the needed conditions on Earth are hot springs similar to ones at Kamchatka. Mineral deposits in these environments under an anoxic atmosphere would have suitable pH (while current pools in an oxygenated atmosphere would not), contain precipitates of photocatalytic sulfide minerals that absorb harmful ultraviolet radiation, have wet-dry cycles that concentrate substrate solutions to concentrations amenable to spontaneous formation of biopolymers created both by chemical reactions in the hydrothermal environment, and by exposure to UV light during transport from vents to adjacent pools that would promote the formation of biomolecules. The hypothesized pre-biotic environments are similar to hydrothermal vents, with additional components that help explain peculiarities of the LUCA.
A phylogenomic and geochemical analysis of proteins plausibly traced to the LUCA shows that the ionic composition of its intracellular fluid is identical to that of hot springs. The LUCA likely was dependent upon synthesized organic matter for its growth. Experiments show that RNA-like polymers can be synthesized in wet-dry cycling and UV light exposure. These polymers were encapsulated in vesicles after condensation. Potential sources of organics at hot springs might have been transport by interplanetary dust particles, extraterrestrial projectiles, or atmospheric or geochemical synthesis. Hot springs could have been abundant in volcanic landmasses during the Hadean.
Temperate surface bodies of water
A mesophilic start in surface bodies of waters hypothesis has evolved from Darwin's concept of a 'warm little pond' and the Oparin-Haldane hypothesis. Freshwater bodies under temperate climates can accumulate prebiotic materials while providing suitable environmental conditions conducive to simple life forms. The climate during the Archaean is still a highly debated topic, as there is uncertainty about what continents, oceans, and the atmosphere looked like then. Atmospheric reconstructions of the Archaean from geochemical proxies and models state that sufficient greenhouse gases were present to maintain surface temperatures between 0-40 °C. Under this assumption, there is a greater abundance of moderate temperature niches in which life could begin.
Strong lines of evidence for mesophily from biomolecular studies include Galtier's G+C nucleotide thermometer. G+C are more abundant in thermophiles due to the added stability of an additional hydrogen bond not present between A+T nucleotides. rRNA sequencing on a diverse range of modern lifeforms show that LUCA's reconstructed G+C content was likely representative of moderate temperatures.
Although most modern phylogenies are thermophilic or hyperthermophilic, it is possible that their widespread diversity today is a product of convergent evolution and horizontal gene transfer rather than an inherited trait from LUCA. The reverse gyrase topoisomerase is found exclusively in thermophiles and hyperthermophiles as it allows for coiling of DNA. The reverse gyrase enzyme requires ATP to function, both of which are complex biomolecules. If an origin of life is hypothesised to involve a simple organism that had not yet evolved a membrane, let alone ATP, this would make the existence of reverse gyrase improbable. Moreover, phylogenetic studies show that reverse gyrase had an archaeal origin, and that it was transferred to bacteria by horizontal gene transfer. This implies that reverse gyrase was not present in the LUCA.
Icy surface bodies of water
Cold-start origin of life theories stem from the idea there may have been cold enough regions on the early Earth that large ice cover could be found. Stellar evolution models predict that the Sun's luminosity was ~25% weaker than it is today. Fuelner states that although this significant decrease in solar energy would have formed an icy planet, there is strong evidence for liquid water to be present, possibly driven by a greenhouse effect. This would create an early Earth with both liquid oceans and icy poles.
Ice melts that form from ice sheets or glaciers melts create freshwater pools, another niche capable of experiencing wet-dry cycles. While these pools that exist on the surface would be exposed to intense UV radiation, bodies of water within and under ice are sufficiently shielded while remaining connected to UV exposed areas through ice cracks. Suggestions of impact melting of ice allow freshwater paired with meteoritic input, a popular vessel for prebiotic components. Near-seawater levels of sodium chloride are found to destabilize fatty acid membrane self-assembly, making freshwater settings appealing for early membranous life.
Icy environments would trade the faster reaction rates that occur in warm environments for increased stability and accumulation of larger polymers. Experiments simulating Europa-like conditions of ~20 °C have synthesised amino acids and adenine, showing that Miller-Urey type syntheses can still occur at cold temperatures. In an RNA world, the ribozyme would have had even more functions than in a later DNA-RNA-protein-world. For RNA to function, it must be able to fold, a process that is hindered by temperatures above 30 °C. While RNA folding in psychrophilic organisms is slower, the process is more successful as hydrolysis is also slower. Shorter nucleotides would not suffer from higher temperatures.
Inside the continental crust
An alternative geological environment has been proposed by the geologist Ulrich Schreiber and the physical chemist Christian Mayer: the continental crust. Tectonic fault zones could present a stable and well-protected environment for long-term prebiotic evolution. Inside these systems of cracks and cavities, water and carbon dioxide present the bulk solvents. Their phase state would depend on the local temperature and pressure conditions and could vary between liquid, gaseous and supercritical. When forming two separate phases (e.g., liquid water and supercritical carbon dioxide in depths of little more than 1 km), the system provides optimal conditions for phase transfer reactions. Concurrently, the contents of the tectonic fault zones are being supplied by a multitude of inorganic educts (e.g., carbon monoxide, hydrogen, ammonia, hydrogen cyanide, nitrogen, and even phosphate from dissolved apatite) and simple organic molecules formed by hydrothermal chemistry (e.g. amino acids, long-chain amines, fatty acids, long-chain aldehydes). Finally, the abundant mineral surfaces provide a rich choice of catalytic activity.
An especially interesting section of the tectonic fault zones is located at a depth of approximately 1000 m. For the carbon dioxide part of the bulk solvent, it provides temperature and pressure conditions near the phase transition point between the supercritical and the gaseous state. This leads to a natural accumulation zone for lipophilic organic molecules that dissolve well in supercritical CO2, but not in its gaseous state, leading to their local precipitation. Periodic pressure variations such as caused by geyser activity or tidal influences result in periodic phase transitions, keeping the local reaction environment in a constant non-equilibrium state. In presence of amphiphilic compounds (such as the long chain amines and fatty acids mentioned above), subsequent generations of vesicles are being formed that are constantly and efficiently being selected for their stability. The resulting structures could provide hydrothermal vents as well as hot springs with raw material for further development.
Homochirality
Main article: Homochirality
Homochirality is the geometric uniformity of materials composed of chiral (non-mirror-symmetric) units. Living organisms use molecules that have the same chirality (handedness): with almost no exceptions, amino acids are left-handed while nucleotides and sugars are right-handed. Chiral molecules can be synthesized, but in the absence of a chiral source or a chiral catalyst, they are formed in a 50/50 (racemic) mixture of both forms. Known mechanisms for the production of non-racemic mixtures from racemic starting materials include: asymmetric physical laws, such as the electroweak interaction; asymmetric environments, such as those caused by circularly polarized light, quartz crystals, or the Earth's rotation, statistical fluctuations during racemic synthesis, and spontaneous symmetry breaking.
Once established, chirality would be selected for. A small bias (enantiomeric excess) in the population can be amplified into a large one by asymmetric autocatalysis, such as in the Soai reaction. In asymmetric autocatalysis, the catalyst is a chiral molecule, which means that a chiral molecule is catalyzing its own production. An initial enantiomeric excess, such as can be produced by polarized light, then allows the more abundant enantiomer to outcompete the other.
Homochirality may have started in outer space, as on the Murchison meteorite the amino acid L-alanine (left-handed) is more than twice as frequent as its D (right-handed) form, and L-glutamic acid is more than three times as abundant as its D counterpart. Amino acids from meteorites show a left-handed bias, whereas sugars show a predominantly right-handed bias: this is the same preference found in living organisms, suggesting an abiogenic origin of these compounds.
In a 2010 experiment by Robert Root-Bernstein, "two D-RNA-oligonucleotides having inverse base sequences (D-CGUA and D-AUGC) and their corresponding L-RNA-oligonucleotides (L-CGUA and L-AUGC) were synthesized and their affinity determined for Gly and eleven pairs of L- and D-amino acids". The results suggest that homochirality, including codon directionality, might have "emerged as a function of the origin of the genetic code".
See also
- Alternative abiogenesis scenarios
- Autopoiesis
- Formamide-based prebiotic chemistry – Scientific efforts aimed at reconstructing the beginnings of life
- Proto-metabolism – Chemical reactions which turn into modern metabolism
- GADV-protein world hypothesis – A hypothetical stage of abiogenesis
- Genetic recombination – Production of offspring with combinations of traits that differ from those found in either parent
- Shadow biosphere – Hypothetical biosphere of Earth
- Manganese metallic nodules
Notes
- The reactions are:
- FeS + H2S → FeS2 + 2H + 2e
- FeS + H2S + CO2 → FeS2 + HCOOH
- The reactions are:
Reaction 1: Fayalite + water → magnetite + aqueous silica + hydrogen- 3Fe2SiO4 + 2H2O → 2Fe3O4 + 3SiO2 + 2H2
- 3Mg2SiO4 + SiO2 + 4H2O → 2Mg3Si2O5(OH)4
- 2Mg2SiO4 + 3H2O → Mg3Si2O5(OH)4 + Mg(OH)2
- 2 Ca2SiO4 + 4 H2O → 3 CaO · 2 SiO2 · 3 H2O + Ca(OH)2
References
- ^ Walker, Sara I.; Packard, N.; Cody, G. D. (13 November 2017). "Re-conceptualizing the origins of life". Philosophical Transactions of the Royal Society A. 375 (2109): 20160337. Bibcode:2017RSPTA.37560337W. doi:10.1098/rsta.2016.0337. PMC 5686397. PMID 29133439.
- ^ "NASA Astrobiology Strategy" (PDF). NASA. 2015. Archived from the original (PDF) on 22 December 2016. Retrieved 24 September 2017.
- Trifonov, Edward N. (17 March 2011). "Vocabulary of Definitions of Life Suggests a Definition". Journal of Biomolecular Structure and Dynamics. 29 (2): 259–266. doi:10.1080/073911011010524992. ISSN 0739-1102. PMID 21875147. S2CID 38476092.
- Voytek, Mary A. (6 March 2021). "About Life Detection". NASA. Archived from the original on 16 August 2021. Retrieved 8 March 2021.
- ^ Witzany, Guenther (2016). "Crucial steps to life: From chemical reactions to code using agents" (PDF). BioSystems. 140: 49–57. Bibcode:2016BiSys.140...49W. doi:10.1016/j.biosystems.2015.12.007. PMID 26723230. S2CID 30962295. Archived (PDF) from the original on 31 October 2018. Retrieved 30 October 2018.
- ^ Howell, Elizabeth (8 December 2014). "How Did Life Become Complex, And Could It Happen Beyond Earth?". Astrobiology Magazine. Archived from the original on 15 February 2018. Retrieved 14 April 2022.
- Oparin, Aleksandr Ivanovich (2003) . The Origin of Life. Translated by Morgulis, Sergius (2 ed.). Mineola, New York: Courier. ISBN 978-0-486-49522-4. Archived from the original on 2 April 2023. Retrieved 16 June 2018.
- ^ Peretó, Juli (2005). "Controversies on the origin of life" (PDF). International Microbiology. 8 (1): 23–31. PMID 15906258. Archived from the original (PDF) on 24 August 2015. Retrieved 1 June 2015.
- Compare: Scharf, Caleb; et al. (18 December 2015). "A Strategy for Origins of Life Research". Astrobiology. 15 (12): 1031–1042. Bibcode:2015AsBio..15.1031S. doi:10.1089/ast.2015.1113. PMC 4683543. PMID 26684503.
What do we mean by the origins of life (OoL)? ... Since the early 20th century the phrase OoL has been used to refer to the events that occurred during the transition from non-living to living systems on Earth, i.e., the origin of terrestrial biology (Oparin, 1924; Haldane, 1929). The term has largely replaced earlier concepts such as abiogenesis (Kamminga, 1980; Fry, 2000).
- ^ Weiss, M. C.; Sousa, F. L.; Mrnjavac, N.; Neukirchen, S.; Roettger, M.; Nelson-Sathi, S.; Martin, W.F. (2016). "The physiology and habitat of the last universal common ancestor" (PDF). Nature Microbiology. 1 (9): 16116. doi:10.1038/NMICROBIOL.2016.116. PMID 27562259. S2CID 2997255. Archived (PDF) from the original on 29 January 2023. Retrieved 21 September 2022.
- Tirard, Stephane (20 April 2015). "Abiogenesis". Encyclopedia of Astrobiology. p. 1. doi:10.1007/978-3-642-27833-4_2-4. ISBN 978-3-642-27833-4.
Thomas Huxley (1825–1895) used the term abiogenesis in an important text published in 1870. He strictly made the difference between spontaneous generation, which he did not accept, and the possibility of the evolution of matter from inert to living, without any influence of life. ... Since the end of the nineteenth century, evolutive abiogenesis means increasing complexity and evolution of matter from inert to living state in the abiotic context of evolution of primitive Earth.
- Luisi, Pier Luigi (2018). The Emergence of Life: From Chemical Origins to Synthetic Biology. Cambridge University Press. p. 416. ISBN 9781108735506.
However, the turning point of non-life to life has never been put into one experimental set up. There are, of course, several hypotheses, and this plethora of ideas means already that we do not have a convincing one.
- Graham, Robert W. (February 1990). "Extraterrestrial Life in the Universe" (PDF). NASA (NASA Technical Memorandum 102363). Lewis Research Center, Cleveland, Ohio. Archived (PDF) from the original on 3 September 2014. Retrieved 2 June 2015.
- Altermann 2009, p. xvii
- Oparin 1953, p. vi
- Warmflash, David; Warmflash, Benjamin (November 2005). "Did Life Come from Another World?". Scientific American. 293 (5): 64–71. Bibcode:2005SciAm.293e..64W. doi:10.1038/scientificamerican1105-64. PMID 16318028.
- Yarus 2010, p. 47
- Ward, Peter; Kirschvink, Joe (2015). A New History of Life: the radical discoveries about the origins and evolution of life on earth. Bloomsbury Press. pp. 39–40. ISBN 978-1-60819-910-5.
- Sheldon 2005
- Lennox 2001, pp. 229–258
- ^ Bernal 1967
- Balme, D. M. (1962). "Development of Biology in Aristotle and Theophrastus: Theory of Spontaneous Generation". Phronesis. 7 (1–2): 91–104. doi:10.1163/156852862X00052.
- Ross 1652
- Dobell 1960
- Bondeson 1999
- Levine, R.; Evers, C. "The Slow Death of Spontaneous Generation (1668-1859)". Archived from the original on 26 April 2008. Retrieved 18 April 2013.
- Oparin 1953, p. 196
- Tyndall 1905, IV, XII (1876), XIII (1878)
- Horneck, Gerda; Klaus, David M.; Mancinelli, Rocco L. (March 2010). "Space Microbiology". Microbiology and Molecular Biology Reviews. 74 (1): 121–156. Bibcode:2010MMBR...74..121H. doi:10.1128/MMBR.00016-09. PMC 2832349. PMID 20197502.
- Wickramasinghe, Chandra (2011). "Bacterial morphologies supporting cometary panspermia: a reappraisal". International Journal of Astrobiology. 10 (1): 25–30. Bibcode:2011IJAsB..10...25W. CiteSeerX 10.1.1.368.4449. doi:10.1017/S1473550410000157. S2CID 7262449.
- Rampelotto, P. H. (2010). "Panspermia: A promising field of research". In: Astrobiology Science Conference. Abs 5224.
- Chang, Kenneth (12 September 2016). "Visions of Life on Mars in Earth's Depths". The New York Times. Archived from the original on 12 September 2016. Retrieved 12 September 2016.
- Aguilera Mochón, Juan Antonio (2016). El origen de la vida en la tierra [The origin of life on Earth] (in Spanish). Spain: RBA. ISBN 978-84-473-8386-3.
- "Letter no. 7471, Charles Darwin to Joseph Dalton Hooker, 1 February (1871)". Darwin Correspondence Project. Archived from the original on 7 July 2020. Retrieved 7 July 2020.
- Priscu, John C. "Origin and Evolution of Life on a Frozen Earth". Arlington County, Virginia: National Science Foundation. Archived from the original on 18 December 2013. Retrieved 1 March 2014.
- Marshall, Michael (11 November 2020). "Charles Darwin's hunch about early life was probably right". BBC News. Archived from the original on 11 November 2020. Retrieved 11 November 2020.
- Bahadur, Krishna (1973). "Photochemical Formation of Self–sustaining Coacervates" (PDF). Proceedings of the Indian National Science Academy. 39 (4): 455–467. doi:10.1016/S0044-4057(75)80076-1. PMID 1242552. Archived from the original (PDF) on 19 October 2013.
- Bahadur, Krishna (1975). "Photochemical Formation of Self-Sustaining Coacervates". Zentralblatt für Bakteriologie, Parasitenkunde, Infektionskrankheiten und Hygiene (Central Journal for Bacteriology, Parasitology, Infectious Diseases and Hygiene). 130 (3): 211–218. doi:10.1016/S0044-4057(75)80076-1. OCLC 641018092. PMID 1242552. Archived from the original on 13 December 2022. Retrieved 13 December 2022.
- Bryson 2004, pp. 300–302
- Bernal 1951
- Martin, William F. (January 2003). "On the origins of cells: a hypothesis for the evolutionary transitions from abiotic geochemistry to chemoautotrophic prokaryotes, and from prokaryotes to nucleated cells". Phil. Trans. R. Soc. Lond. A. 358 (1429): 59–83. doi:10.1098/rstb.2002.1183. PMC 1693102. PMID 12594918.
- Bernal, John Desmond (September 1949). "The Physical Basis of Life". Proceedings of the Physical Society, Section A. 62 (9): 537–558. Bibcode:1949PPSA...62..537B. doi:10.1088/0370-1298/62/9/301. S2CID 83754271.
- Miller, Stanley L. (15 May 1953). "A Production of Amino Acids Under Possible Primitive Earth Conditions". Science. 117 (3046): 528–529. Bibcode:1953Sci...117..528M. doi:10.1126/science.117.3046.528. PMID 13056598.
- Parker, Eric T.; Cleaves, Henderson J.; Dworkin, Jason P.; et al. (5 April 2011). "Primordial synthesis of amines and amino acids in a 1958 Miller H2S-rich spark discharge experiment". PNAS. 108 (14): 5526–5531. Bibcode:2011PNAS..108.5526P. doi:10.1073/pnas.1019191108. PMC 3078417. PMID 21422282.
- Bernal 1967, p. 143
- ^ Cleaves, H. James; Chalmers, John H.; Lazcano, Antonio; et al. (April 2008). "A Reassessment of Prebiotic Organic Synthesis in Neutral Planetary Atmospheres". Origins of Life and Evolution of Biospheres. 38 (2): 105–115. Bibcode:2008OLEB...38..105C. doi:10.1007/s11084-007-9120-3. PMID 18204914. S2CID 7731172.
- Chyba, Christopher F. (13 May 2005). "Rethinking Earth's Early Atmosphere". Science. 308 (5724): 962–963. doi:10.1126/science.1113157. PMID 15890865. S2CID 93303848.
- Barton et al. 2007, pp. 93–95
- Bada & Lazcano 2009, pp. 56–57
- Bada, Jeffrey L.; Lazcano, Antonio (2 May 2003). "Prebiotic Soup – Revisiting the Miller Experiment" (PDF). Science. 300 (5620): 745–746. doi:10.1126/science.1085145. PMID 12730584. S2CID 93020326. Archived (PDF) from the original on 4 March 2016. Retrieved 13 June 2015.
- Madau, Piero; Dickinson, Mark (18 August 2014). "Cosmic Star-Formation History". Annual Review of Astronomy and Astrophysics. 52 (1): 415–486. arXiv:1403.0007. Bibcode:2014ARA&A..52..415M. doi:10.1146/annurev-astro-081811-125615. S2CID 658354. Archived from the original on 1 July 2022. Retrieved 8 December 2023.
- Marigo, Paola (6 July 2020). "Carbon star formation as seen through the non-monotonic initial–final mass relation". Nature Astronomy. 152 (11): 1102–1110. arXiv:2007.04163. Bibcode:2020NatAs...4.1102M. doi:10.1038/s41550-020-1132-1. S2CID 220403402. Archived from the original on 16 February 2023. Retrieved 7 July 2020.
- "WMAP- Life in the Universe". Archived from the original on 29 January 2023. Retrieved 27 September 2019.
- "Formation of Solar Systems: Solar Nebular Theory". University of Massachusetts Amherst. Archived from the original on 27 September 2019. Retrieved 27 September 2019.
- "Age of the Earth". United States Geological Survey. 9 July 2007. Archived from the original on 23 December 2005. Retrieved 10 January 2006.
- Dalrymple 2001, pp. 205–221
- Fesenkov 1959, p. 9
- Bottke, W. F.; Vokrouhlický, D.; Marchi, S.; Swindle, T.; Scott, E. R. D.; Weirich, J. R.; Levison, H. (17 April 2015). "Dating the Moon-forming impact event with asteroidal meteorites". Science. 348 (6232): 321–323. Bibcode:2015Sci...348..321B. doi:10.1126/science.aaa0602. PMID 25883354. S2CID 206632612.
- Kasting, James F. (12 February 1993). "Earth's Early Atmosphere" (PDF). Science. 259 (5097): 920–926. Bibcode:1993Sci...259..920K. doi:10.1126/science.11536547. PMID 11536547. S2CID 21134564. Archived from the original (PDF) on 10 October 2015. Retrieved 28 July 2015.
- ^ Follmann, Hartmut; Brownson, Carol (November 2009). "Darwin's warm little pond revisited: from molecules to the origin of life". Naturwissenschaften. 96 (11): 1265–1292. Bibcode:2009NW.....96.1265F. doi:10.1007/s00114-009-0602-1. PMID 19760276. S2CID 23259886.
- Morse, John (September 1998). "Hadean Ocean Carbonate Geochemistry". Aquatic Geochemistry. 4 (3/4): 301–319. Bibcode:1998MinM...62.1027M. doi:10.1023/A:1009632230875. S2CID 129616933.
- Sleep, Norman H.; Zahnle, Kevin J.; Lupu, Roxana E. (13 September 2014). "Terrestrial aftermath of the Moon-forming impact". Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences. 372 (2024): 20130172. Bibcode:2014RSPTA.37230172S. doi:10.1098/rsta.2013.0172. PMID 25114303. S2CID 6902632.
- Morse, John W.; Mackenzie, Fred T. (1998). "[No title found]". Aquatic Geochemistry. 4 (3/4): 301–319. doi:10.1023/A:1009632230875. S2CID 129616933. Archived from the original on 31 January 2024. Retrieved 8 December 2023.
- Crowley, James L.; Myers, John S.; Sylvester, Paul J; Cox, Richard A. (May 2005). "Detrital Zircon from the Jack Hills and Mount Narryer, Western Australia: Evidence for Diverse >4.0 Ga Source Rocks". The Journal of Geology. 113 (3): 239–263. Bibcode:2005JG....113..239C. doi:10.1086/428804. S2CID 140715676. Archived from the original on 16 December 2023. Retrieved 8 December 2023.
- Wilde, Simon A.; Valley, John W.; Peck, William H.; Graham, Colin M. (11 January 2001). "Evidence from detrital zircons for the existence of continental crust and oceans on the Earth 4.4 Gyr ago" (PDF). Nature. 409 (6817): 175–178. Bibcode:2001Natur.409..175W. doi:10.1038/35051550. PMID 11196637. S2CID 4319774. Archived (PDF) from the original on 5 June 2015. Retrieved 3 June 2015.
- Korenaga, Jun (December 2008). "Plate tectonics, flood basalts and the evolution of Earth's oceans". Terra Nova. 20 (6): 419–439. Bibcode:2008TeNov..20..419K. doi:10.1111/j.1365-3121.2008.00843.x. S2CID 36766331.
- Rosing, Minik T.; Bird, Dennis K.; Sleep, Norman H.; et al. (22 March 2006). "The rise of continents – An essay on the geologic consequences of photosynthesis". Palaeogeography, Palaeoclimatology, Palaeoecology. 232 (2–4): 99–113. Bibcode:2006PPP...232...99R. doi:10.1016/j.palaeo.2006.01.007. Archived (PDF) from the original on 14 July 2015. Retrieved 8 June 2015.
- Tera, Fouad; Papanastassiou, D.A.; Wasserburg, G.J. (April 1974). "Isotopic evidence for a terminal lunar cataclysm". Earth and Planetary Science Letters. 22 (1): 1–21. Bibcode:1974E&PSL..22....1T. doi:10.1016/0012-821x(74)90059-4. Archived from the original on 31 January 2024.
- Stoffler, D. (1 January 2006). "Cratering History and Lunar Chronology". Reviews in Mineralogy and Geochemistry. 60 (1): 519–596. Bibcode:2006RvMG...60..519S. doi:10.2138/rmg.2006.60.05. Archived from the original on 31 January 2024.
- Sleep, Norman H.; Zahnle, Kevin J.; Kasting, James F.; Morowitz, Harold J. (December 1989). "Annihilation of ecosystems by large asteroid impacts on the early Earth". Nature. 342 (6246): 139–142. Bibcode:1989Natur.342..139S. doi:10.1038/342139a0. PMID 11536616. S2CID 1137852. Archived from the original on 31 January 2024.
- Fassett, Caleb I.; Minton, David A. (23 June 2013). "Impact bombardment of the terrestrial planets and the early history of the Solar System". Nature Geoscience. 6 (7): 520–524. Bibcode:2013NatGe...6..520F. doi:10.1038/ngeo1841. Archived from the original on 31 January 2024.
- Abramov, Oleg; Mojzsis, Stephen J. (May 2009). "Microbial habitability of the Hadean Earth during the late heavy bombardment". Nature. 459 (7245): 419–422. Bibcode:2009Natur.459..419A. doi:10.1038/nature08015. PMID 19458721. S2CID 3304147. Archived from the original on 31 January 2024.
- Boehnke, Patrick; Harrison, T. Mark (12 September 2016). "Illusory Late Heavy Bombardments". Proceedings of the National Academy of Sciences. 113 (39): 10802–10806. Bibcode:2016PNAS..11310802B. doi:10.1073/pnas.1611535113. PMC 5047187. PMID 27621460.
- Zellner, Nicolle E. B. (3 May 2017). "Cataclysm No More: New Views on the Timing and Delivery of Lunar Impactors". Origins of Life and Evolution of Biospheres. 47 (3): 261–280. arXiv:1704.06694. Bibcode:2017OLEB...47..261Z. doi:10.1007/s11084-017-9536-3. PMC 5602003. PMID 28470374.
- Lowe, Donald R.; Byerly, Gary R. (1 April 2018). "The terrestrial record of Late Heavy Bombardment". New Astronomy Reviews. 81: 39–61. Bibcode:2018NewAR..81...39L. doi:10.1016/j.newar.2018.03.002.
- Davies 1999, p. 155
- Bock & Goode 1996
- ^ Dodd, Matthew S.; Papineau, Dominic; Grenne, Tor; et al. (1 March 2017). "Evidence for early life in Earth's oldest hydrothermal vent precipitates". Nature. 543 (7643): 60–64. Bibcode:2017Natur.543...60D. doi:10.1038/nature21377. PMID 28252057. Archived from the original on 8 September 2017. Retrieved 2 March 2017.
- Ohtomo, Yoko; Kakegawa, Takeshi; Ishida, Akizumi; et al. (January 2014). "Evidence for biogenic graphite in early Archaean Isua metasedimentary rocks". Nature Geoscience. 7 (1): 25–28. Bibcode:2014NatGe...7...25O. doi:10.1038/ngeo2025.
- Noffke, Nora; Christian, Daniel; Wacey, David; Hazen, Robert M. (16 November 2013). "Microbially Induced Sedimentary Structures Recording an Ancient Ecosystem in the ca. 3.48 Gyo Dresser Formation, Pilbara, Western Australia". Astrobiology. 13 (12): 1103–1124. Bibcode:2013AsBio..13.1103N. doi:10.1089/ast.2013.1030. PMC 3870916. PMID 24205812.
- Davies 1999
- Hassenkam, T.; Andersson, M. P.; Dalby, K. N.; Mackenzie, D. M. A.; Rosing, M.T. (2017). "Elements of Eoarchean life trapped in mineral inclusions". Nature. 548 (7665): 78–81. Bibcode:2017Natur.548...78H. doi:10.1038/nature23261. PMID 28738409. S2CID 205257931.
- O'Donoghue, James (21 August 2011). "Oldest reliable fossils show early life was a beach". New Scientist. 211: 13. doi:10.1016/S0262-4079(11)62064-2. Archived from the original on 30 June 2015.
- Wacey, David; Kilburn, Matt R.; Saunders, Martin; et al. (October 2011). "Microfossils of sulphur-metabolizing cells in 3.4-billion-year-old rocks of Western Australia". Nature Geoscience. 4 (10): 698–702. Bibcode:2011NatGe...4..698W. doi:10.1038/ngeo1238.
- Bell, Elizabeth A.; Boehnke, Patrick; Harrison, T. Mark; Mao, Wendy L. (24 November 2015). "Potentially biogenic carbon preserved in a 4.1 billion-year-old zircon". Proceedings of the National Academy of Sciences. 112 (47): 14518–14521. Bibcode:2015PNAS..11214518B. doi:10.1073/pnas.1517557112. PMC 4664351. PMID 26483481.
- Baumgartner, Rafael; Van Kranendonk, Martin; Wacey, David; et al. (2019). "Nano−porous pyrite and organic matter in 3.5-billion-year-old stromatolites record primordial life" (PDF). Geology. 47 (11): 1039–1043. Bibcode:2019Geo....47.1039B. doi:10.1130/G46365.1. S2CID 204258554. Archived (PDF) from the original on 5 December 2020. Retrieved 10 January 2021.
- Djokic, Tara; Van Kranendonk, Martin J.; Campbell, Kathleen A.; Walter, Malcolm R.; Ward, Colin R. (9 May 2017). "Earliest signs of life on land preserved in ca. 3.5 Gao hot spring deposits". Nature Communications. 8: 15263. Bibcode:2017NatCo...815263D. doi:10.1038/ncomms15263. PMC 5436104. PMID 28486437.
- Betts, Holly C.; Puttick, Mark N.; Clark, James W.; Williams, Tom A.; Donoghue, Philip C. J.; Pisani, Davide (20 August 2018). "Integrated genomic and fossil evidence illuminates life's early evolution and eukaryote origin". Nature Ecology & Evolution. 2 (10): 1556–1562. Bibcode:2018NatEE...2.1556B. doi:10.1038/s41559-018-0644-x. PMC 6152910. PMID 30127539.
- Landau, Elizabeth (12 October 2016). "Building Blocks of Life's Building Blocks Come From Starlight". NASA. Archived from the original on 13 October 2016. Retrieved 13 October 2016.
- ^ Ehrenfreund, Pascale; Cami, Jan (December 2010). "Cosmic carbon chemistry: from the interstellar medium to the early Earth". Cold Spring Harbor Perspectives in Biology. 2 (12): a002097. doi:10.1101/cshperspect.a002097. PMC 2982172. PMID 20554702.
- Geballe, Thomas R.; Najarro, Francisco; Figer, Donald F.; et al. (10 November 2011). "Infrared diffuse interstellar bands in the Galactic Centre region". Nature. 479 (7372): 200–202. arXiv:1111.0613. Bibcode:2011Natur.479..200G. doi:10.1038/nature10527. PMID 22048316. S2CID 17223339.
- Klyce 2001
- ^ Hoover, Rachel (21 February 2014). "Need to Track Organic Nano-Particles Across the Universe? NASA's Got an App for That". Ames Research Center. NASA. Archived from the original on 6 September 2015. Retrieved 22 June 2015.
- Goncharuk, Vladislav V.; Zui, O. V. (February 2015). "Water and carbon dioxide as the main precursors of organic matter on Earth and in space". Journal of Water Chemistry and Technology. 37 (1): 2–3. Bibcode:2015JWCT...37....2G. doi:10.3103/S1063455X15010026. S2CID 97965067.
- Abou Mrad, Ninette; Vinogradoff, Vassilissa; Duvernay, Fabrice; et al. (2015). "Laboratory experimental simulations: Chemical evolution of the organic matter from interstellar and cometary ice analogs". Bulletin de la Société Royale des Sciences de Liège. 84: 21–32. Bibcode:2015BSRSL..84...21A. Archived from the original on 13 April 2015. Retrieved 6 April 2015.
- Oba, Yasuhiro (26 April 2022). "Identifying the wide diversity of extraterrestrial purine and pyrimidine nucleobases in carbonaceous meteorites". Nature Communications. 13 (2008): 2008. Bibcode:2022NatCo..13.2008O. doi:10.1038/s41467-022-29612-x. PMC 9042847. PMID 35473908. S2CID 248402205.
- "'Life chemical' detected in comet". BBC News. London. 18 August 2009. Archived from the original on 25 May 2015. Retrieved 23 June 2015.
- Thompson, William Reid; Murray, B. G.; Khare, Bishun Narain; Sagan, Carl (30 December 1987). "Coloration and darkening of methane clathrate and other ices by charged particle irradiation: Applications to the outer solar system". Journal of Geophysical Research. 92 (A13): 14933–14947. Bibcode:1987JGR....9214933T. doi:10.1029/JA092iA13p14933. PMID 11542127.
- Goldman, Nir; Tamblyn, Isaac (20 June 2013). "Prebiotic Chemistry within a Simple Impacting Icy Mixture". Journal of Physical Chemistry A. 117 (24): 5124–5131. Bibcode:2013JPCA..117.5124G. doi:10.1021/jp402976n. PMID 23639050. S2CID 5144843. Archived from the original on 21 July 2018. Retrieved 29 August 2019.
- https://www.astronomy.com/science/how-much-dust-falls-on-earth-each-year-does-it-affect-our-planets-gravity/
- "NASA Ames PAH IR Spectroscopic Database". NASA. Archived from the original on 29 June 2015. Retrieved 17 June 2015.
- ^ Hudgins, Douglas M.; Bauschlicher, Charles W. Jr.; Allamandola, Louis J. (10 October 2005). "Variations in the Peak Position of the 6.2 μm Interstellar Emission Feature: A Tracer of N in the Interstellar Polycyclic Aromatic Hydrocarbon Population". The Astrophysical Journal. 632 (1): 316–332. Bibcode:2005ApJ...632..316H. CiteSeerX 10.1.1.218.8786. doi:10.1086/432495. S2CID 7808613.
- ^ Des Marais, David J.; Allamandola, Louis J.; Sandford, Scott; et al. (2009). "Cosmic Distribution of Chemical Complexity". Ames Research Center. Mountain View, California: NASA. Archived from the original on 27 February 2014. Retrieved 24 June 2015.
- ^ Carey, Bjorn (18 October 2005). "Life's Building Blocks 'Abundant in Space'". Space.com. Watsonville, California: Imaginova. Archived from the original on 26 June 2015. Retrieved 23 June 2015.
- García-Hernández, Domingo. A.; Manchado, Arturo; García-Lario, Pedro; et al. (20 November 2010). "Formation of Fullerenes in H-Containing Planetary Nebulae". The Astrophysical Journal Letters. 724 (1): L39 – L43. arXiv:1009.4357. Bibcode:2010ApJ...724L..39G. doi:10.1088/2041-8205/724/1/L39. S2CID 119121764.
- Gudipati, Murthy S.; Yang, Rui (1 September 2012). "In-situ Probing of Radiation-induced Processing of Organics in Astrophysical Ice Analogs – Novel Laser Desorption Laser Ionization Time-of-flight Mass Spectroscopic Studies". The Astrophysical Journal Letters. 756 (1): L24. Bibcode:2012ApJ...756L..24G. doi:10.1088/2041-8205/756/1/L24. S2CID 5541727.
- ^ Gallori, Enzo (June 2011). "Astrochemistry and the origin of genetic material". Rendiconti Lincei. 22 (2): 113–118. doi:10.1007/s12210-011-0118-4. S2CID 96659714. "Paper presented at the Symposium 'Astrochemistry: molecules in space and time' (Rome, 4–5 November 2010), sponsored by Fondazione 'Guido Donegani', Accademia Nazionale dei Lincei."
- Martins, Zita (February 2011). "Organic Chemistry of Carbonaceous Meteorites". Elements. 7 (1): 35–40. Bibcode:2011Eleme...7...35M. doi:10.2113/gselements.7.1.35.
- Martins, Zita; Botta, Oliver; Fogel, Marilyn L.; et al. (15 June 2008). "Extraterrestrial nucleobases in the Murchison meteorite". Earth and Planetary Science Letters. 270 (1–2): 130–136. arXiv:0806.2286. Bibcode:2008E&PSL.270..130M. doi:10.1016/j.epsl.2008.03.026. S2CID 14309508.
- Callahan, Michael P.; Smith, Karen E.; Cleaves, H. James II; et al. (23 August 2011). "Carbonaceous meteorites contain a wide range of extraterrestrial nucleobases". PNAS. 108 (34): 13995–13998. Bibcode:2011PNAS..10813995C. doi:10.1073/pnas.1106493108. PMC 3161613. PMID 21836052.
- Steigerwald, John (8 August 2011). "NASA Researchers: DNA Building Blocks Can Be Made in Space". Goddard Space Flight Center. NASA. Archived from the original on 23 June 2015. Retrieved 23 June 2015.
- Kwok, Sun; Zhang, Yong (3 November 2011). "Mixed aromatic–aliphatic organic nanoparticles as carriers of unidentified infrared emission features". Nature. 479 (7371): 80–83. Bibcode:2011Natur.479...80K. doi:10.1038/nature10542. PMID 22031328. S2CID 4419859.
- Jørgensen, Jes K.; Favre, Cécile; Bisschop, Suzanne E.; et al. (2012). "Detection of the simplest sugar, glycolaldehyde, in a solar-type protostar with ALMA" (PDF). The Astrophysical Journal Letters. 757 (1): L4. arXiv:1208.5498. Bibcode:2012ApJ...757L...4J. doi:10.1088/2041-8205/757/1/L4. S2CID 14205612. Archived (PDF) from the original on 24 September 2015. Retrieved 23 June 2015.
- Furukawa, Yoshihiro; Chikaraishi, Yoshito; Ohkouchi, Naohiko; et al. (13 November 2019). "Extraterrestrial ribose and other sugars in primitive meteorites". PNAS. 116 (49): 24440–24445. Bibcode:2019PNAS..11624440F. doi:10.1073/pnas.1907169116. PMC 6900709. PMID 31740594.
- Oró, Joan; Kimball, Aubrey P. (February 1962). "Synthesis of purines under possible primitive earth conditions: II. Purine intermediates from hydrogen cyanide". Archives of Biochemistry and Biophysics. 96 (2): 293–313. doi:10.1016/0003-9861(62)90412-5. PMID 14482339.
- Cleaves II, Henderson (2010). "The origin of the biologically coded amino acids". Journal of Theoretical Biology. 263 (4): 490–498. Bibcode:2010JThBi.263..490C. doi:10.1016/j.jtbi.2009.12.014. PMID 20034500.
- Green, N. J., Russell, D. A., Tanner, S. H., Sutherland, J. D. (2023). "Prebiotic Synthesis of N-Formylaminonitriles and Derivatives in Formamide". Journal of the American Chemical Society. 145 (19): 10533–10541. Bibcode:2023JAChS.14510533G. doi:10.1021/jacs.2c13306. PMC 10197134. PMID 37146260.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Breslow, R. (1959). "On the Mechanism of the Formose Reaction". Tetrahedron Letters. 1 (21): 22–26. doi:10.1016/S0040-4039(01)99487-0.
- Oró, Joan (16 September 1961). "Mechanism of Synthesis of Adenine from Hydrogen Cyanide under Possible Primitive Earth Conditions". Nature. 191 (4794): 1193–1194. Bibcode:1961Natur.191.1193O. doi:10.1038/1911193a0. PMID 13731264. S2CID 4276712.
- ^ Saladino, Raffaele; Crestini, Claudia; Pino, Samanta; et al. (March 2012). "Formamide and the origin of life" (PDF). Physics of Life Reviews. 9 (1): 84–104. Bibcode:2012PhLRv...9...84S. doi:10.1016/j.plrev.2011.12.002. hdl:2108/85168. PMID 22196896. Archived (PDF) from the original on 27 January 2023. Retrieved 29 August 2019.
- ^ Saladino, Raffaele; Botta, Giorgia; Pino, Samanta; et al. (July 2012). "From the one-carbon amide formamide to RNA all the steps are prebiotically possible". Biochimie. 94 (7): 1451–1456. doi:10.1016/j.biochi.2012.02.018. hdl:11573/515604. PMID 22738728.
- Marlaire, Ruth, ed. (3 March 2015). "NASA Ames Reproduces the Building Blocks of Life in Laboratory". Ames Research Center. NASA. Archived from the original on 5 March 2015. Retrieved 5 March 2015.
- Ferus, Martin; Nesvorný, David; Šponer, Jiří; et al. (2015). "High-energy chemistry of formamide: A unified mechanism of nucleobase formation". PNAS. 112 (3): 657–662. Bibcode:2015PNAS..112..657F. doi:10.1073/pnas.1412072111. PMC 4311869. PMID 25489115.
- Basile, Brenda; Lazcano, Antonio; Oró, Joan (1984). "Prebiotic syntheses of purines and pyrimidines". Advances in Space Research. 4 (12): 125–131. Bibcode:1984AdSpR...4l.125B. doi:10.1016/0273-1177(84)90554-4. PMID 11537766.
- Orgel, Leslie E. (August 2004). "Prebiotic Adenine Revisited: Eutectics and Photochemistry". Origins of Life and Evolution of Biospheres. 34 (4): 361–369. Bibcode:2004OLEB...34..361O. doi:10.1023/B:ORIG.0000029882.52156.c2. PMID 15279171. S2CID 4998122.
- Robertson, Michael P.; Miller, Stanley L. (29 June 1995). "An efficient prebiotic synthesis of cytosine and uracil". Nature. 375 (6534): 772–774. Bibcode:1995Natur.375..772R. doi:10.1038/375772a0. PMID 7596408. S2CID 4351012.
- Fox, Douglas (1 February 2008). "Did Life Evolve in Ice?". Discover. Archived from the original on 30 June 2008. Retrieved 3 July 2008.
- Levy, Matthew; Miller, Stanley L.; Brinton, Karen; Bada, Jeffrey L. (June 2000). "Prebiotic Synthesis of Adenine and Amino Acids Under Europa-like Conditions". Icarus. 145 (2): 609–613. Bibcode:2000Icar..145..609L. doi:10.1006/icar.2000.6365. PMID 11543508.
- Menor-Salván, César; Ruiz-Bermejo, Marta; Guzmán, Marcelo I.; et al. (20 April 2009). "Synthesis of Pyrimidines and Triazines in Ice: Implications for the Prebiotic Chemistry of Nucleobases". Chemistry: A European Journal. 15 (17): 4411–4418. doi:10.1002/chem.200802656. PMID 19288488.
- Roy, Debjani; Najafian, Katayoun; von Ragué Schleyer, Paul (30 October 2007). "Chemical evolution: The mechanism of the formation of adenine under prebiotic conditions". PNAS. 104 (44): 17272–17277. Bibcode:2007PNAS..10417272R. doi:10.1073/pnas.0708434104. PMC 2077245. PMID 17951429.
- ^ Frenkel-Pinter, Moran; Samanta, Mousumi; Ashkenasy, Gonen; Leman, Luke J. (10 June 2020). "Prebiotic Peptides: Molecular Hubs in the Origin of Life". Chemical Reviews. 120 (11): 4707–4765. Bibcode:2020ChRv..120.4707F. doi:10.1021/acs.chemrev.9b00664. ISSN 0009-2665. PMID 32101414.
- Campbell, Thomas D.; Febrian, Rio; McCarthy, Jack T.; Kleinschmidt, Holly E.; Forsythe, Jay G.; Bracher, Paul J. (2019). "Prebiotic condensation through wet–dry cycling regulated by deliquescence". Nature Communications. 10 (1): 4508. Bibcode:2019NatCo..10.4508C. doi:10.1038/s41467-019-11834-1. PMC 6778215. PMID 31586058.
- Marshall-Bowman, Karina; Ohara, Shohei; Sverjensky, Dimitri A.; Hazen, Robert M.; Cleaves, H. James (October 2010). "Catalytic peptide hydrolysis by mineral surface: Implications for prebiotic chemistry". Geochimica et Cosmochimica Acta. 74 (20): 5852–5861. Bibcode:2010GeCoA..74.5852M. doi:10.1016/j.gca.2010.07.009. ISSN 0016-7037.
- Matreux, Thomas; Aikkila, Paula; Scheu, Bettina; Braun, Dieter; Mast, Christof B. (April 2024). "Heat flows enrich prebiotic building blocks and enhance their reactivity". Nature. 628 (8006): 110–116. Bibcode:2024Natur.628..110M. doi:10.1038/s41586-024-07193-7. ISSN 1476-4687. PMC 10990939. PMID 38570715.
- Paecht-Horowitz, Mella (1 January 1974). "The possible role of clays in prebiotic peptide synthesis". Origins of Life. 5 (1): 173–187. Bibcode:1974OrLi....5..173P. doi:10.1007/BF00927022. ISSN 1573-0875. PMID 4842069.
- Krasnokutski, S. A.; Chuang, K.-J.; Jäger, C.; Ueberschaar, N.; Henning, Th. (10 February 2022). "A pathway to peptides in space through the condensation of atomic carbon". Nature Astronomy. 6 (3): 381–386. arXiv:2202.12170. Bibcode:2022NatAs...6..381K. doi:10.1038/s41550-021-01577-9. ISSN 2397-3366.
- Krasnokutski, Serge A.; Jäger, Cornelia; Henning, Thomas; Geffroy, Claude; Remaury, Quentin B.; Poinot, Pauline (19 April 2024). "Formation of extraterrestrial peptides and their derivatives". Science Advances. 10 (16): eadj7179. arXiv:2405.00744. Bibcode:2024SciA...10J7179K. doi:10.1126/sciadv.adj7179. ISSN 2375-2548. PMC 11023503. PMID 38630826.
- Lancet, Doron (30 December 2014). "Systems Prebiology-Studies of the origin of Life". The Lancet Lab. Rehovot, Israel: Department of Molecular Genetics; Weizmann Institute of Science. Archived from the original on 26 June 2015. Retrieved 26 June 2015.
- Segré, Daniel; Ben-Eli, Dafna; Deamer, David W.; Lancet, Doron (February 2001). "The Lipid World" (PDF). Origins of Life and Evolution of Biospheres. 31 (1–2): 119–145. Bibcode:2001OLEB...31..119S. doi:10.1023/A:1006746807104. PMID 11296516. S2CID 10959497. Archived (PDF) from the original on 26 June 2015.
- ^ Chen, Irene A.; Walde, Peter (July 2010). "From Self-Assembled Vesicles to Protocells". Cold Spring Harbor Perspectives in Biology. 2 (7): a002170. doi:10.1101/cshperspect.a002170. PMC 2890201. PMID 20519344.
- Eigen, Manfred; Schuster, Peter (November 1977). "The Hypercycle. A Principle of Natural Self-Organization. Part A: Emergence of the Hypercycle" (PDF). Naturwissenschaften. 64 (11): 541–65. Bibcode:1977NW.....64..541E. doi:10.1007/bf00450633. PMID 593400. S2CID 42131267. Archived from the original (PDF) on 3 March 2016.
- Eigen, Manfred; Schuster, Peter (1978). "The Hypercycle. A Principle of Natural Self-Organization. Part B: The Abstract Hypercycle" (PDF). Naturwissenschaften. 65 (1): 7–41. Bibcode:1978NW.....65....7E. doi:10.1007/bf00420631. S2CID 1812273. Archived from the original (PDF) on 3 March 2016.
- Eigen, Manfred; Schuster, Peter (July 1978). "The Hypercycle. A Principle of Natural Self-Organization. Part C: The Realistic Hypercycle" (PDF). Naturwissenschaften. 65 (7): 341–369. Bibcode:1978NW.....65..341E. doi:10.1007/bf00439699. S2CID 13825356. Archived from the original (PDF) on 16 June 2016.
- Markovitch, Omer; Lancet, Doron (Summer 2012). "Excess Mutual Catalysis Is Required for Effective Evolvability". Artificial Life. 18 (3): 243–266. doi:10.1162/artl_a_00064. PMID 22662913. S2CID 5236043.
- Tessera, Marc (2011). "Origin of Evolution versus Origin of Life: A Shift of Paradigm". International Journal of Molecular Sciences. 12 (6): 3445–3458. doi:10.3390/ijms12063445. PMC 3131571. PMID 21747687. Special Issue: "Origin of Life 2011"
- "Protocells". Exploring Life's Origins: A Virtual Exhibit. Arlington County, Virginia: National Science Foundation. Archived from the original on 28 February 2014. Retrieved 13 September 2023.
- ^ Chen, Irene A. (8 December 2006). "The Emergence of Cells During the Origin of Life". Science. 314 (5805): 1558–1559. doi:10.1126/science.1137541. PMID 17158315.
- Zimmer, Carl (26 June 2004). "What Came Before DNA?". Discover. Archived from the original on 19 March 2014.
- Onsager, Lars (1931). "Reciprocal Relations in Irreversible Processes I". Physical Review. 37 (4): 405. Bibcode:1931PhRv...37..405O. doi:10.1103/PhysRev.37.405.
- Onsager, Lars (1931). "Reciprocal Relations in Irreversible Processes II". Physical Review. 38 (12): 2265. Bibcode:1931PhRv...38.2265O. doi:10.1103/PhysRev.38.2265.
- Prigogine, Ilya (1967). An Introduction to the Thermodynamics of Irreversible Processes. New York: Wiley.
- Shapiro, Robert (June 2007). "A Simpler Origin for Life". Scientific American. 296 (6): 46–53. Bibcode:2007SciAm.296f..46S. doi:10.1038/scientificamerican0607-46. PMID 17663224. Archived from the original on 14 June 2015.
- Chang 2007
- ^ Lane, Nick (2015). The Vital Question: Why Is Life The Way It Is?. Profile Books. pp. 129–140. ISBN 978-1-78125-036-5.
- Jordan, Sean F.; Nee, Eloise; Lane, Nick (6 December 2019). "Isoprenoids enhance the stability of fatty acid membranes at the emergence of life potentially leading to an early lipid divide". Interface Focus. 9 (6): 20190067. doi:10.1098/rsfs.2019.0067. PMC 6802135. PMID 31641436.
- Mayer, Christian; Schreiber, Ulrich; Dávila, María J.; Schmitz, Oliver J.; Bronja, Amela; Meyer, Martin; Klein, Julia; Meckelmann, Sven W. (2018). "Molecular Evolution in a Peptide-Vesicle System". Life. 8 (2): 16. Bibcode:2018Life....8...16M. doi:10.3390/life8020016. ISSN 2075-1729. PMC 6027363. PMID 29795023.
- Mayer, Christian; Schreiber, Ulrich; Dávila, María J. (1 June 2015). "Periodic Vesicle Formation in Tectonic Fault Zones—an Ideal Scenario for Molecular Evolution". Origins of Life and Evolution of Biospheres. 45 (1): 139–148. Bibcode:2015OLEB...45..139M. doi:10.1007/s11084-015-9411-z. ISSN 1573-0875. PMC 4457167. PMID 25716918.
- Dávila, María J.; Mayer, Christian (2022). "Membrane Structure Obtained in an Experimental Evolution Process". Life. 12 (2): 145. Bibcode:2022Life...12..145D. doi:10.3390/life12020145. ISSN 2075-1729. PMC 8875328. PMID 35207433.
- Mayer, Christian (2022). "Spontaneous Formation of Functional Structures in Messy Environments". Life. 12 (5): 720. Bibcode:2022Life...12..720M. doi:10.3390/life12050720. ISSN 2075-1729. PMC 9148140. PMID 35629387.
- Dávila, María J.; Mayer, Christian (2023). "Structural Phenomena in a Vesicle Membrane Obtained through an Evolution Experiment: A Study Based on MD Simulations". Life. 13 (8): 1735. Bibcode:2023Life...13.1735D. doi:10.3390/life13081735. ISSN 2075-1729. PMC 10455627. PMID 37629592.
- Mayer, Christian (18 January 2020). "Life in The Context of Order and Complexity". Life. 10 (1): 5. Bibcode:2020Life...10....5M. doi:10.3390/life10010005. ISSN 2075-1729. PMC 7175320. PMID 31963637.
- Sharov, Alexei A.; Gordon, Richard (2018). "Life Before Earth". Habitability of the Universe Before Earth: Life Before Earth. Astrobiology Exploring Life on Earth and Beyond. Academic Press. pp. 265–296. doi:10.1016/B978-0-12-811940-2.00011-3. ISBN 978-0-12-811940-2. S2CID 117048600. Archived from the original on 30 April 2022. Retrieved 30 April 2022.
- Ladyman, J.; Lambert, J.; Weisner, K. B. (2013). "What is a Complex System?". European Journal of the Philosophy of Science. 3: 33–67. doi:10.1007/s13194-012-0056-8. S2CID 18787276.
- Esposito, M.; Lindenberg, Katja; Van den Broeck, C. (2010). "Entropy production as correlation between system and reservoir". New Journal of Physics. 12 (1): 013013. arXiv:0908.1125. Bibcode:2010NJPh...12a3013E. doi:10.1088/1367-2630/12/1/013013. S2CID 26657293.
- Bar-Nun, A.; Bar-Nun, N.; Bauer, S. H.; Sagan, Carl (24 April 1970). "Shock Synthesis of Amino Acids in Simulated Primitive Environments". Science. 168 (3930): 470–473. Bibcode:1970Sci...168..470B. doi:10.1126/science.168.3930.470. PMID 5436082. S2CID 42467812.
- Anbar, Michael (27 September 1968). "Cavitation during Impact of Liquid Water on Water: Geochemical Implications". Science. 161 (3848): 1343–1344. Bibcode:1968Sci...161.1343A. doi:10.1126/science.161.3848.1343. PMID 17831346.
- Dharmarathne, Leena; Grieser, Franz (7 January 2016). "Formation of Amino Acids on the Sonolysis of Aqueous Solutions Containing Acetic Acid, Methane, or Carbon Dioxide, in the Presence of Nitrogen Gas". The Journal of Physical Chemistry A. 120 (2): 191–199. Bibcode:2016JPCA..120..191D. doi:10.1021/acs.jpca.5b11858. PMID 26695890.
- Patehebieke, Yeersen; Zhao, Ze-Run; Wang, Su; Xu, Hao-Xing; Chen, Qian-Qian; Wang, Xiao (2021). "Cavitation as a plausible driving force for the prebiotic formation of N9 purine nucleosides". Cell Reports Physical Science. 2 (3): 100375. Bibcode:2021CRPS....200375P. doi:10.1016/j.xcrp.2021.100375. S2CID 233662126.
- Kalson, Natan-Haim; Furman, David; Zeiri, Yehuda (11 September 2017). "Cavitation-Induced Synthesis of Biogenic Molecules on Primordial Earth". ACS Central Science. 3 (9): 1041–1049. doi:10.1021/acscentsci.7b00325. PMC 5620973. PMID 28979946. S2CID 21409351.
- Muller, Anthonie W. J. (1995). "Were the first organisms heat engines? A new model for biogenesis and the early evolution of biological energy conversion". Progress in Biophysics and Molecular Biology. 63 (2): 193–231. doi:10.1016/0079-6107(95)00004-7. PMID 7542789.
- Muller, Anthonie W. J.; Schulze-Makuch, Dirk (2006). "Thermal energy and the origin of life". Origins of Life and Evolution of Biospheres. 36 (2): 77–189. Bibcode:2006OLEB...36..177M. doi:10.1007/s11084-005-9003-4. PMID 16642267. S2CID 22179552.
- Junge, Wolfgang; Nelson, Nathan (2 June 2015). "ATP Synthase". Annual Review of Biochemistry. 84 (1): 631–657. doi:10.1146/annurev-biochem-060614-034124. PMID 25839341.
- ^ Damer, Bruce; Deamer, David (1 April 2020). "The Hot Spring Hypothesis for an Origin of Life". Astrobiology. 20 (4): 429–452. Bibcode:2020AsBio..20..429D. doi:10.1089/ast.2019.2045. PMC 7133448. PMID 31841362.
- d'Ischia, Marco; Manini, Paola; Moracci, Marco; et al. (21 August 2019). "Astrochemistry and Astrobiology: Materials Science in Wonderland?". International Journal of Molecular Sciences. 20 (17): 4079. doi:10.3390/ijms20174079. PMC 6747172. PMID 31438518.
- Carey, Bjorn (18 October 2005). "Life's Building Blocks 'Abundant in Space'". Space.com. Retrieved 3 March 2014.
- Hudgins, Douglas M.; Bauschlicher, Jr, Charles W.; Allamandola, L. J. (10 October 2005). "Variations in the Peak Position of the 6.2 μm Interstellar Emission Feature: A Tracer of N in the Interstellar Polycyclic Aromatic Hydrocarbon Population". Astrophysical Journal. 632 (1): 316–332. Bibcode:2005ApJ...632..316H. CiteSeerX 10.1.1.218.8786. doi:10.1086/432495. S2CID 7808613.
- Allamandola, Louis; et al. (13 April 2011). "Cosmic Distribution of Chemical Complexity". NASA. Archived from the original on 27 February 2014. Retrieved 3 March 2014.
- Clavin, Whitney (10 February 2015). "Why Comets Are Like Deep Fried Ice Cream". NASA. Retrieved 10 February 2015.
- Platts, Simon Nicholas, "The PAH World - Discotic polynuclear aromatic compounds as a mesophase scaffolding at the origin of life"
- Benner, S. A.; Bell, E. A.; Biondi, E.; Brasser, R.; Carell, T.; Kim, H.-J.; Mojzsis, S. J.; Omran, A.; Pasek, M. A.; Trail, D. (2020). "When Did Life Likely Emerge on Earth in an RNA-First Process?". ChemSystemsChem. 2 (2). arXiv:1908.11327. Bibcode:2020CSysC...2...35B. doi:10.1002/syst.201900035.
- * Copley, Shelley D.; Smith, Eric; Morowitz, Harold J. (December 2007). "The origin of the RNA world: Co-evolution of genes and metabolism" (PDF). Bioorganic Chemistry. 35 (6): 430–443. doi:10.1016/j.bioorg.2007.08.001. PMID 17897696. Archived (PDF) from the original on 5 September 2013. Retrieved 8 June 2015.
The proposal that life on Earth arose from an RNA world is widely accepted.
- Orgel, Leslie E. (April 2003). "Some consequences of the RNA world hypothesis". Origins of Life and Evolution of Biospheres. 33 (2): 211–218. Bibcode:2003OLEB...33..211O. doi:10.1023/A:1024616317965. PMID 12967268. S2CID 32779859.
It now seems very likely that our familiar DNA/RNA/protein world was preceded by an RNA world...
- Robertson & Joyce 2012: "There is now strong evidence indicating that an RNA World did indeed exist before DNA- and protein-based life."
- Neveu, Kim & Benner 2013: " has broad support within the community today."
- Orgel, Leslie E. (April 2003). "Some consequences of the RNA world hypothesis". Origins of Life and Evolution of Biospheres. 33 (2): 211–218. Bibcode:2003OLEB...33..211O. doi:10.1023/A:1024616317965. PMID 12967268. S2CID 32779859.
- ^ Robertson, Michael P.; Joyce, Gerald F. (May 2012). "The origins of the RNA world". Cold Spring Harbor Perspectives in Biology. 4 (5): a003608. doi:10.1101/cshperspect.a003608. PMC 3331698. PMID 20739415.
- ^ Cech, Thomas R. (July 2012). "The RNA Worlds in Context". Cold Spring Harbor Perspectives in Biology. 4 (7): a006742. doi:10.1101/cshperspect.a006742. PMC 3385955. PMID 21441585.
- Pearce, Ben K. D.; Pudritz, Ralph E.; Semenov, Dmitry A.; Henning, Thomas K. (24 October 2017). "Origin of the RNA world: The fate of nucleobases in warm little ponds". PNAS. 114 (43): 11327–11332. arXiv:1710.00434. Bibcode:2017PNAS..11411327P. doi:10.1073/pnas.1710339114. PMC 5664528. PMID 28973920.
- Yarus, Michael (April 2011). "Getting Past the RNA World: The Initial Darwinian Ancestor". Cold Spring Harbor Perspectives in Biology. 3 (4): a003590. doi:10.1101/cshperspect.a003590. PMC 3062219. PMID 20719875.
- Voet & Voet 2004, p. 29
- Fox, George.E. (9 June 2010). "Origin and evolution of the ribosome". Cold Spring Harbor Perspectives in Biology. 2 (9(a003483)): a003483. doi:10.1101/cshperspect.a003483. PMC 2926754. PMID 20534711.
- Neveu, Marc; Kim, Hyo-Joong; Benner, Steven A. (22 April 2013). "The 'Strong' RNA World Hypothesis: Fifty Years Old". Astrobiology. 13 (4): 391–403. Bibcode:2013AsBio..13..391N. doi:10.1089/ast.2012.0868. PMID 23551238.
- Gilbert, Walter (20 February 1986). "Origin of life: The RNA world". Nature. 319 (6055): 618. Bibcode:1986Natur.319..618G. doi:10.1038/319618a0. S2CID 8026658.
- Orgel, Leslie E. (October 1994). "The origin of life on Earth". Scientific American. 271 (4): 76–83. Bibcode:1994SciAm.271d..76O. doi:10.1038/scientificamerican1094-76. PMID 7524147.
- Lincoln, Tracey A.; Joyce, Gerald F. (27 February 2009). "Self-Sustained Replication of an RNA Enzyme". Science. 323 (5918): 1229–1232. Bibcode:2009Sci...323.1229L. doi:10.1126/science.1167856. PMC 2652413. PMID 19131595.
- Joyce, Gerald F. (2009). "Evolution in an RNA world". Cold Spring Harbor Perspectives in Biology. 74 (Evolution: The Molecular Landscape): 17–23. doi:10.1101/sqb.2009.74.004. PMC 2891321. PMID 19667013.
- Szostak, Jack W. (5 February 2015). "The Origins of Function in Biological Nucleic Acids, Proteins, and Membranes". Chevy Chase, Maryland: Howard Hughes Medical Institute. Archived from the original on 14 July 2015. Retrieved 16 June 2015.
- Bernstein, Harris; Byerly, Henry C.; Hopf, Frederick A.; et al. (June 1983). "The Darwinian Dynamic". The Quarterly Review of Biology. 58 (2): 185–207. doi:10.1086/413216. JSTOR 2828805. S2CID 83956410.
- Michod 1999
- Palasek, Stan (23 May 2013). "Primordial RNA Replication and Applications in PCR Technology". arXiv:1305.5581v1 .
- Vlassov, Alexander V.; Kazakov, Sergei A.; Johnston, Brian H.; et al. (August 2005). "The RNA World on Ice: A New Scenario for the Emergence of RNA Information". Journal of Molecular Evolution. 61 (2): 264–273. Bibcode:2005JMolE..61..264V. doi:10.1007/s00239-004-0362-7. PMID 16044244. S2CID 21096886.
- Nussinov, Mark D.; Otroshchenko, Vladimir A.; Santoli, Salvatore (1997). "The emergence of the non-cellular phase of life on the fine-grained clayish particles of the early Earth's regolith". BioSystems. 42 (2–3): 111–118. Bibcode:1997BiSys..42..111N. doi:10.1016/S0303-2647(96)01699-1. PMID 9184757.
- Kühnlein, Alexandra; Lanzmich, Simon A.; Brun, Dieter (2 March 2021). "tRNA sequences can assemble into a replicator". eLife. 10. doi:10.7554/eLife.63431. PMC 7924937. PMID 33648631.
- Noller, Harry F. (April 2012). "Evolution of protein synthesis from an RNA world". Cold Spring Harbor Perspectives in Biology. 4 (4): a003681. Bibcode:2012CSHPB...4.3681N. doi:10.1101/cshperspect.a003681. PMC 3312679. PMID 20610545.
- Koonin, Eugene V. (31 May 2007). "The cosmological model of eternal inflation and the transition from chance to biological evolution in the history of life". Biology Direct. 2: 15. doi:10.1186/1745-6150-2-15. PMC 1892545. PMID 17540027.
- ^ Tamura, K.; Alexander, R. W. (1 May 2004). "Peptide synthesis through evolution". Cellular and Molecular Life Sciences. 61 (11): 1317–1330. doi:10.1007/s00018-004-3449-9. ISSN 1420-9071. PMC 11138682. PMID 15170510.
- Tamura, Koji; Schimmel, Paul (22 July 2003). "Peptide synthesis with a template-like RNA guide and aminoacyl phosphate adaptors". Proceedings of the National Academy of Sciences. 100 (15): 8666–8669. Bibcode:2003PNAS..100.8666T. doi:10.1073/pnas.1432909100. ISSN 0027-8424. PMC 166369. PMID 12857953.
- ^ Pressman, Abe D.; Liu, Ziwei; Janzen, Evan; Blanco, Celia; Müller, Ulrich F.; Joyce, Gerald F.; Pascal, Robert; Chen, Irene A. (17 April 2019). "Mapping a Systematic Ribozyme Fitness Landscape Reveals a Frustrated Evolutionary Network for Self-Aminoacylating RNA". Journal of the American Chemical Society. 141 (15): 6213–6223. Bibcode:2019JAChS.141.6213P. doi:10.1021/jacs.8b13298. ISSN 0002-7863. PMC 6548421. PMID 30912655.
- Tyagi, Sanjay; Ponnamperuma, Cyril (1 May 1990). "Nonrandomness in prebiotic peptide synthesis". Journal of Molecular Evolution. 30 (5): 391–399. Bibcode:1990JMolE..30..391T. doi:10.1007/BF02101111. ISSN 1432-1432. PMID 2111852.
- Keefe, Anthony D.; Szostak, Jack W. (2001). "Functional proteins from a random-sequence library". Nature. 410 (6829): 715–718. Bibcode:2001Natur.410..715K. doi:10.1038/35070613. PMC 4476321. PMID 11287961.
- Tong, Cher Ling; Lee, Kun-Hwa; Seelig, Burckhard (June 2021). "De novo proteins from random sequences through in vitro evolution". Current Opinion in Structural Biology. 68: 129–134. Bibcode:2021COStB..68..129T. doi:10.1016/j.sbi.2020.12.014. PMC 8222087. PMID 33517151.
- Boone, David R.; Castenholz, Richard W.; Garrity, George M., eds. (2001). The Archaea and the Deeply Branching and Phototrophic Bacteria. Bergey's Manual of Systematic Bacteriology. Springer. ISBN 978-0-387-21609-6. Archived from the original on 25 December 2014.
- Woese, C. R.; Fox, G. E. (1977). "Phylogenetic structure of the prokaryotic domain: the primary kingdoms". PNAS. 7 (11): 5088–5090. Bibcode:1977PNAS...74.5088W. doi:10.1073/pnas.74.11.5088. PMC 432104. PMID 270744.
- Valas, R. E.; Bourne, P. E. (2011). "The origin of a derived superkingdom: how a gram-positive bacterium crossed the desert to become an archaeon". Biology Direct. 6: 16. doi:10.1186/1745-6150-6-16. PMC 3056875. PMID 21356104.
- Cavalier-Smith, Thomas (2006). "Rooting the tree of life by transition analyses". Biology Direct. 1: 19. doi:10.1186/1745-6150-1-19. PMC 1586193. PMID 16834776.
- "Early life liked it hot". Nature. 535 (7613): 468. 2016. doi:10.1038/535468b. S2CID 49905802.
- Gogarten, Johann Peter; Deamer, David (25 November 2016). "Is LUCA a thermophilic progenote?". Nature Microbiology. 1 (12): 16229. doi:10.1038/nmicrobiol.2016.229. PMID 27886195. S2CID 205428194. Archived from the original on 3 April 2020. Retrieved 21 September 2022.
- Catchpole, Ryan; Forterre, Patrick (2019). "The evolution of Reverse Gyrase suggests a non-hyperthermophilic Last Universal Common Ancestor". Molecular Biology and Evolution. 36 (12): 2737–2747. doi:10.1093/molbev/msz180. PMC 6878951. PMID 31504731. Archived from the original on 27 January 2023. Retrieved 18 September 2022.
- Berkemer, Sarah J.; McGlynn, Shawn E (8 August 2020). "A New Analysis of Archaea–Bacteria Domain Separation: Variable Phylogenetic Distance and the Tempo of Early Evolution". Molecular Biology and Evolution. 37 (8): 2332–2340. doi:10.1093/molbev/msaa089. PMC 7403611. PMID 32316034. Archived from the original on 27 January 2023. Retrieved 21 September 2022.
- Koonin, E. V. (2003). "Comparative genomics, minimal gene-sets and the last universal common ancestor". Nature Reviews. Microbiology. 1 (2): 127–136. doi:10.1038/nrmicro751. PMID 15035042.
- Hoffmann, Geoffrey W. (25 June 1974). "On the origin of the genetic code and the stability of the translation apparatus". Journal of Molecular Biology. 86 (2): 349–362. doi:10.1016/0022-2836(74)90024-2. PMID 4414916.
- Orgel, Leslie E. (April 1963). "The Maintenance of the Accuracy of Protein Synthesis and its Relevance to Ageing". PNAS. 49 (4): 517–521. Bibcode:1963PNAS...49..517O. doi:10.1073/pnas.49.4.517. PMC 299893. PMID 13940312.
- Hoffmann, Geoffrey W. (October 1975). "The Stochastic Theory of the Origin of the Genetic Code". Annual Review of Physical Chemistry. 26: 123–144. Bibcode:1975ARPC...26..123H. doi:10.1146/annurev.pc.26.100175.001011.
- Harrison, Stuart A.; Palmeira, Raquel Nunes; Halpern, Aaron; Lane, Nick (1 November 2022). "A biophysical basis for the emergence of the genetic code in protocells". Biochimica et Biophysica Acta (BBA) - Bioenergetics. 1863 (8): 148597. doi:10.1016/j.bbabio.2022.148597. PMID 35868450. S2CID 250707510.
- Harrison, Stuart A.; Lane, Nick (12 December 2018). "Life as a guide to prebiotic nucleotide synthesis". Nature Communications. 9 (1): 5176. Bibcode:2018NatCo...9.5176H. doi:10.1038/s41467-018-07220-y. PMC 6289992. PMID 30538225.
- Cantine, Marjorie D.; Fournier, Gregory P. (1 March 2018). "Environmental Adaptation from the Origin of Life to the Last Universal Common Ancestor". Origins of Life and Evolution of Biospheres. 48 (1): 35–54. Bibcode:2018OLEB...48...35C. doi:10.1007/s11084-017-9542-5. hdl:1721.1/114219. PMID 28685374. S2CID 254888920. Archived from the original on 31 January 2024.
- Mat, Wai-Kin (1 May 2008). "The genomics of LUCA" (PDF). Frontiers in Bioscience. 13 (14): 5605–5613. doi:10.2741/3103. PMID 18508609. Archived (PDF) from the original on 16 June 2022. Retrieved 8 December 2023.
- Brasier, M. D. (2012). Secret Chambers: The Inside Story of Cells and Complex Life. Oxford University Press. p. 298.
- Ward, Peter & Kirschvink, Joe, op cit, p. 42
- ^ Colín-García, M.; Heredia, A.; Cordero, G.; et al. (2016). "Hydrothermal vents and prebiotic chemistry: a review". Boletín de la Sociedad Geológica Mexicana. 68 (3): 599–620. doi:10.18268/BSGM2016v68n3a13. Archived from the original on 18 August 2017.
- Schirber, Michael (24 June 2014). "Hydrothermal Vents Could Explain Chemical Precursors to Life". NASA Astrobiology: Life in the Universe. NASA. Archived from the original on 29 November 2014. Retrieved 19 June 2015.
- ^ Martin, William; Russell, Michael J. (29 January 2003). "On the origins of cells: a hypothesis for the evolutionary transitions from abiotic geochemistry to chemoautotrophic prokaryotes, and from prokaryotes to nucleated cells". Philosophical Transactions of the Royal Society B. 358 (1429): 59–83, discussion 83–85. doi:10.1098/rstb.2002.1183. PMC 1693102. PMID 12594918.
- Lane 2009
- Usher, Oli (27 April 2015). "Chemistry of seabed's hot vents could explain emergence of life" (Press release). University College London. Archived from the original on 20 June 2015. Retrieved 19 June 2015.
- Roldan, Alberto; Hollingsworth, Nathan; Roffey, Anna; et al. (May 2015). "Bio-inspired CO2 conversion by iron sulfide catalysts under sustainable conditions". Chemical Communications. 51 (35): 7501–7504. doi:10.1039/C5CC02078F. PMID 25835242. Archived from the original on 20 June 2015. Retrieved 19 June 2015.
- Baross, J. A.; Hoffman, S. E. (1985). "Submarine hydrothermal vents and associated gradient environments as sites for the origin and evolution of life". Origins of Life and Evolution of Biospheres. 15 (4): 327–345. Bibcode:1985OrLi...15..327B. doi:10.1007/bf01808177. S2CID 4613918.
- Russell, M. J.; Hall, A. J. (1997). "The emergence of life from iron monosulphide bubbles at a submarine hydrothermal redox and pH front". Journal of the Geological Society. 154 (3): 377–402. Bibcode:1997JGSoc.154..377R. doi:10.1144/gsjgs.154.3.0377. PMID 11541234. S2CID 24792282.
- Amend, J. P.; LaRowe, D. E.; McCollom, T. M.; Shock, E. L. (2013). "The energetics of organic synthesis inside and outside the cell". Philosophical Transactions of the Royal Society B. 368 (1622): 20120255. doi:10.1098/rstb.2012.0255. PMC 3685458. PMID 23754809.
- Shock, E. L.; Boyd, E. S. (2015). "Geomicrobiology and microbial geochemistry:principles of geobiochemistry". Elements. 11: 389–394. doi:10.2113/gselements.11.6.395.
- Martin, W.; Russell, M. J. (2007). "On the origin of biochemistry at an alkaline hydrothermal vent". Philosophical Transactions of the Royal Society B. 362 (1486): 1887–1925. doi:10.1098/rstb.2006.1881. PMC 2442388. PMID 17255002.
- Lane, Nick; Martin, William F. (21 December 2012). "The Origin of Membrane Bioenergetics". Cell. 151 (7): 1406–1416. doi:10.1016/j.cell.2012.11.050. PMID 23260134. S2CID 15028935.
- Baaske, Philipp; Weinert, Franz M.; Duhr, Stefan; Lemke, Kono H.; Russell, Michael J.; Braun, Dieter (29 May 2007). "Extreme accumulation of nucleotides in simulated hydrothermal pore systems". Proceedings of the National Academy of Sciences. 104 (22): 9346–9351. Bibcode:2007PNAS..104.9346B. doi:10.1073/pnas.0609592104. PMC 1890497. PMID 17494767.
- Nunes Palmeira, Raquel; Colnaghi, Marco; Harrison, Stuart A.; Pomiankowski, Andrew; Lane, Nick (9 November 2022). "The limits of metabolic heredity in protocells". Proceedings of the Royal Society B: Biological Sciences. 289 (1986). doi:10.1098/rspb.2022.1469. PMC 9653231. PMID 36350219.
- Woese, Carl R. (1987). "Bacterial evolution". Microbiological Reviews. 51.2 (1987) (2): 221–271. doi:10.1128/mr.51.2.221-271.1987. PMC 373105. PMID 2439888. S2CID 734579.
- Russell, Michael J.; Hall, Allan J. (2006), "The onset and early evolution of life", Evolution of Early Earth's Atmosphere, Hydrosphere, and Biosphere - Constraints from Ore Deposits, Geological Society of America, doi:10.1130/2006.1198(01) (inactive 11 December 2024), ISBN 9780813711980, archived from the original on 31 January 2024
{{citation}}: CS1 maint: DOI inactive as of December 2024 (link) - Boussau, Bastien; Blanquart, Samuel; Necsulea, Anamaria; Lartillot, Nicolas; Gouy, Manolo (December 2008). "Parallel adaptations to high temperatures in the Archaean eon". Nature. 456 (7224): 942–945. Bibcode:2008Natur.456..942B. doi:10.1038/nature07393. PMID 19037246. S2CID 4348746. Archived from the original on 16 December 2023. Retrieved 8 December 2023.
- ^ Galtier, Nicolas; Tourasse, Nicolas; Gouy, Manolo (8 January 1999). "A Nonhyperthermophilic Common Ancestor to Extant Life Forms". Science. 283 (5399): 220–221. doi:10.1126/science.283.5399.220. PMID 9880254. Archived from the original on 31 January 2024. Retrieved 8 December 2023.
- Kelley, Deborah S.; Karson, Jeffrey A.; Blackman, Donna K.; Früh-Green, Gretchen L.; Butterfield, David A.; Lilley, Marvin D.; Olson, Eric J.; Schrenk, Matthew O.; Roe, Kevin K.; Lebon, Geoff T.; Rivizzigno, Pete (July 2001). "An off-axis hydrothermal vent field near the Mid-Atlantic Ridge at 30° N". Nature. 412 (6843): 145–149. Bibcode:2001Natur.412..145K. doi:10.1038/35084000. PMID 11449263. S2CID 4407013. Archived from the original on 31 January 2024.
- ^ Ehrenfreund, P.; Irvine, W.; Becker, L.; Blank, J.; Brucato, J. R.; et al. (August 2002). "Astrophysical and astrochemical insights into the origin of life". Reports on Progress in Physics. 65 (10): 1427. Bibcode:2002RPPh...65.1427E. doi:10.1088/0034-4885/65/10/202. S2CID 250904448.
- Chyba, C.F.; Chyba, C.F.; Hand, K.P. (2006). "Comets and Prebiotic Organic Molecules on Early Earth". Comets and the Origin and Evolution of Life. Advances in Astrobiology and Biogeophysics. Springer Berlin Heidelberg. pp. 169–206. doi:10.1007/3-540-33088-7_6. ISBN 978-3-540-33086-8. Archived from the original on 31 January 2024.
- Chatterjee, Sankar (2023), Chatterjee, Sankar (ed.), "The Cradle of Life", From Stardust to First Cells: The Origin and Evolution of Early Life, Cham: Springer International Publishing, pp. 43–66, doi:10.1007/978-3-031-23397-5_6, ISBN 978-3-031-23397-5, archived from the original on 31 January 2024
- Deamer, David W. (7 February 2019). "Prospects for Life on Other Planets". Assembling Life. Oxford University Press. doi:10.1093/oso/9780190646387.003.0017. ISBN 978-0-19-064638-7. Archived from the original on 31 January 2024.
- Pearce, Ben K. D.; Pudritz, Ralph E.; Semenov, Dmitry A.; Henning, Thomas K. (2 October 2017). "Origin of the RNA world: The fate of nucleobases in warm little ponds". Proceedings of the National Academy of Sciences. 114 (43): 11327–11332. arXiv:1710.00434. Bibcode:2017PNAS..11411327P. doi:10.1073/pnas.1710339114. PMC 5664528. PMID 28973920.
- ^ Deamer, David (10 February 2021). "Where Did Life Begin? Testing Ideas in Prebiotic Analogue Conditions". Life. 11 (2): 134. Bibcode:2021Life...11..134D. doi:10.3390/life11020134. PMC 7916457. PMID 33578711.
- Jordan, Sean F.; Rammu, Hanadi; Zheludev, Ivan N.; Hartley, Andrew M.; Maréchal, Amandine; Lane, Nick (2019). "Promotion of protocell self-assembly from mixed amphiphiles at the origin of life". Nature Ecology & Evolution. 3 (12): 1705–1714. Bibcode:2019NatEE...3.1705J. doi:10.1038/s41559-019-1015-y. PMID 31686020.
- Korenaga, Jun (November 2021). "Was There Land on the Early Earth?". Life. 11 (11): 1142. Bibcode:2021Life...11.1142K. doi:10.3390/life11111142. PMC 8623345. PMID 34833018.
- Powner, Matthew W.; Gerland, Béatrice; Sutherland, John D. (May 2009). "Synthesis of activated pyrimidine ribonucleotides in prebiotically plausible conditions". Nature. 459 (7244): 239–242. Bibcode:2009Natur.459..239P. doi:10.1038/nature08013. PMID 19444213. S2CID 4412117. Archived from the original on 12 November 2023. Retrieved 8 December 2023.
- Zahnle, Kevin; Arndt, Nick; Cockell, Charles; Halliday, Alex; Nisbet, Euan; Selsis, Franck; Sleep, Norman H. (1 March 2007). "Emergence of a Habitable Planet". Space Science Reviews. 129 (1): 35–78. Bibcode:2007SSRv..129...35Z. doi:10.1007/s11214-007-9225-z. S2CID 12006144. Archived from the original on 31 January 2024.
- Woese, C R (June 1987). "Bacterial evolution". Microbiological Reviews. 51 (2): 221–271. doi:10.1128/mr.51.2.221-271.1987. PMC 373105. PMID 2439888.
- ^ Mulkidjanian, Armen Y.; Bychkov, Andrew Yu.; Dibrova, Daria V.; Galperin, Michael Y.; Koonin, Eugene V. (3 April 2012). "Origin of first cells at terrestrial, anoxic geothermal fields". Proceedings of the National Academy of Sciences. 109 (14): E821-30. Bibcode:2012PNAS..109E.821M. doi:10.1073/pnas.1117774109. PMC 3325685. PMID 22331915.
- Chandru, Kuhan; Guttenberg, Nicholas; Giri, Chaitanya; et al. (31 May 2018). "Simple prebiotic synthesis of high diversity dynamic combinatorial polyester libraries". Communications Chemistry. 1 (1): 30. Bibcode:2018CmChe...1...30C. doi:10.1038/s42004-018-0031-1.
- Forsythe, Jay G.; Yu, Sheng-Sheng; Mamajanov, Irena; et al. (17 August 2015). "Ester-Mediated Amide Bond Formation Driven by Wet–Dry Cycles: A Possible Path to Polypeptides on the Prebiotic Earth". Angewandte Chemie International Edition in English. 54 (34): 9871–9875. Bibcode:2015AngCh..54.9871F. doi:10.1002/anie.201503792. PMC 4678426. PMID 26201989.
- Patel, Bhavesh H.; Percivalle, Claudia; Ritson, Dougal J.; Duffy, Colm. D.; Sutherland, John D. (16 March 2015). "Common origins of RNA, protein and lipid precursors in a cyanosulfidic protometabolism". Nature Chemistry. 7 (4): 301–307. Bibcode:2015NatCh...7..301P. doi:10.1038/nchem.2202. PMC 4568310. PMID 25803468.
- Catling, David C.; Zahnle, Kevin J. (28 February 2020). "The Archean atmosphere". Science Advances. 6 (9): eaax1420. Bibcode:2020SciA....6.1420C. doi:10.1126/sciadv.aax1420. PMC 7043912. PMID 32133393.
- Miller, Stanley L.; Lazcano, Antonio (December 1995). "The origin of life?did it occur at high temperatures?". Journal of Molecular Evolution. 41 (6): 689–692. Bibcode:1995JMolE..41..689M. doi:10.1007/bf00173146. hdl:2060/19980211388. PMID 11539558. S2CID 25141419. Archived from the original on 31 January 2024.
- Forterre, Patrick; Bergerat, Agnes; Lopex-Garcia, Purificacion (May 1996). "The unique DNA topology and DNA topoisomerases of hyperthermophilic archaea". FEMS Microbiology Reviews. 18 (2–3): 237–248. doi:10.1111/j.1574-6976.1996.tb00240.x. PMID 8639331. S2CID 6001830.
- Brochier-Armanet, Céline; Forterre, Patrick (May 2006). "Widespread distribution of archaeal reverse gyrase in thermophilic bacteria suggests a complex history of vertical inheritance and lateral gene transfers". Archaea. 2 (2): 83–93. doi:10.1155/2006/582916. PMC 2686386. PMID 17350929.
- Feulner, Georg (June 2012). "The faint young Sun problem". Reviews of Geophysics. 50 (2). arXiv:1204.4449. Bibcode:2012RvGeo..50.2006F. doi:10.1029/2011RG000375. S2CID 119248267. Archived from the original on 8 December 2023. Retrieved 8 December 2023.
- Bada, J. L.; Bigham, C.; Miller, S. L. (15 February 1994). "Impact melting of frozen oceans on the early Earth: Implications for the origin of life". Proceedings of the National Academy of Sciences. 91 (4): 1248–1250. Bibcode:1994PNAS...91.1248B. doi:10.1073/pnas.91.4.1248. PMC 43134. PMID 11539550.
- Monnard, Pierre-Alain; Apel, Charles L.; Kanavarioti, Anastassia; Deamer, David W. (June 2002). "Influence of Ionic Inorganic Solutes on Self-Assembly and Polymerization Processes Related to Early Forms of Life: Implications for a Prebiotic Aqueous Medium". Astrobiology. 2 (2): 139–152. Bibcode:2002AsBio...2..139M. doi:10.1089/15311070260192237. PMID 12469365. Archived from the original on 31 January 2024. Retrieved 8 December 2023.
- Attwater, James; Wochner, Aniela; Holliger, Philipp (December 2013). "In-ice evolution of RNA polymerase ribozyme activity". Nature Chemistry. 5 (12): 1011–1018. Bibcode:2013NatCh...5.1011A. doi:10.1038/nchem.1781. PMC 3920166. PMID 24256864.
- Levy, Matthew; Miller, Stanley L.; Brinton, Karen; Bada, Jeffrey L. (1 June 2000). "Prebiotic Synthesis of Adenine and Amino Acids Under Europa-like Conditions". Icarus. 145 (2): 609–613. Bibcode:2000Icar..145..609L. doi:10.1006/icar.2000.6365. PMID 11543508.
- Moulton, Vincent; Gardner, Paul P.; Pointon, Robert F.; Creamer, Lawrence K.; Jameson, Geoffrey B.; Penny, David (1 October 2000). "RNA Folding Argues Against a Hot-Start Origin of Life". Journal of Molecular Evolution. 51 (4): 416–421. Bibcode:2000JMolE..51..416M. doi:10.1007/s002390010104. PMID 11040293. S2CID 20787323. Archived from the original on 31 January 2024.
- Zemora, Georgeta; Waldsich, Christina (November 2010). "RNA folding in living cells". RNA Biology. 7 (6): 634–641. doi:10.4161/rna.7.6.13554. PMC 3073324. PMID 21045541.
- Schreiber, Ulrich; Locker-Grütjen, Oliver; Mayer, Christian (2012). "Hypothesis: Origin of Life in Deep-Reaching Tectonic Faults". Origins of Life and Evolution of Biospheres. 42 (1): 47–54. Bibcode:2012OLEB...42...47S. doi:10.1007/s11084-012-9267-4. ISSN 0169-6149. PMID 22373604.
- Schreiber, Ulrich; Mayer, Christian; Schmitz, Oliver J.; Rosendahl, Pia; Bronja, Amela; Greule, Markus; Keppler, Frank; Mulder, Ines; Sattler, Tobias; Schöler, Heinz F. (14 June 2017). Stüeken, Eva Elisabeth (ed.). "Organic compounds in fluid inclusions of Archean quartz—Analogues of prebiotic chemistry on early Earth". PLOS ONE. 12 (6): e0177570. Bibcode:2017PLoSO..1277570S. doi:10.1371/journal.pone.0177570. ISSN 1932-6203. PMC 5470662. PMID 28614348.
- Großmann, Yildiz; Schreiber, Ulrich; Mayer, Christian; Schmitz, Oliver J. (21 June 2022). "Aliphatic Aldehydes in the Earth's Crust—Remains of Prebiotic Chemistry?". Life. 12 (7): 925. Bibcode:2022Life...12..925G. doi:10.3390/life12070925. ISSN 2075-1729. PMC 9319801. PMID 35888015.
- Mayer, Christian; Schreiber, Ulrich; Dávila, María (7 January 2017). "Selection of Prebiotic Molecules in Amphiphilic Environments". Life. 7 (1): 3. Bibcode:2017Life....7....3M. doi:10.3390/life7010003. ISSN 2075-1729. PMC 5370403. PMID 28067845.
- Mayer, Christian; Schreiber, Ulrich; Dávila, María J. (2015). "Periodic Vesicle Formation in Tectonic Fault Zones—an Ideal Scenario for Molecular Evolution". Origins of Life and Evolution of Biospheres. 45 (1–2): 139–148. Bibcode:2015OLEB...45..139M. doi:10.1007/s11084-015-9411-z. ISSN 0169-6149. PMC 4457167. PMID 25716918.
- Mayer, Christian; Schreiber, Ulrich; Dávila, María; Schmitz, Oliver; Bronja, Amela; Meyer, Martin; Klein, Julia; Meckelmann, Sven (24 May 2018). "Molecular Evolution in a Peptide-Vesicle System". Life. 8 (2): 16. Bibcode:2018Life....8...16M. doi:10.3390/life8020016. ISSN 2075-1729. PMC 6027363. PMID 29795023.
- ^ Plasson, Raphaël; Kondepudi, Dilip K.; Bersini, Hugues; et al. (August 2007). "Emergence of homochirality in far-from-equilibrium systems: Mechanisms and role in prebiotic chemistry". Chirality. 19 (8): 589–600. doi:10.1002/chir.20440. PMID 17559107. "Special Issue: Proceedings from the Eighteenth International Symposium on Chirality (ISCD-18), Busan, Korea, 2006"
- Chaichian, Rojas & Tureanu 2014, pp. 353–364
- Jafarpour, Farshid; Biancalani, Tommaso; Goldenfeld, Nigel (2017). "Noise-induced symmetry breaking far from equilibrium and the emergence of biological homochirality" (PDF). Physical Review E. 95 (3): 032407. Bibcode:2017PhRvE..95c2407J. doi:10.1103/PhysRevE.95.032407. PMID 28415353. Archived from the original on 2 April 2023. Retrieved 29 August 2019.
- Jafarpour, Farshid; Biancalani, Tommaso; Goldenfeld, Nigel (2015). "Noise-induced mechanism for biological homochirality of early life self-replicators". Physical Review Letters. 115 (15): 158101. arXiv:1507.00044. Bibcode:2015PhRvL.115o8101J. doi:10.1103/PhysRevLett.115.158101. PMID 26550754. S2CID 9775791.
- Frank, F. C. (1953). "On spontaneous asymmetric synthesis". Biochimica et Biophysica Acta. 11 (4): 459–463. doi:10.1016/0006-3002(53)90082-1. PMID 13105666.
- Clark, Stuart (July–August 1999). "Polarized Starlight and the Handedness of Life". American Scientist. 87 (4): 336. Bibcode:1999AmSci..87..336C. doi:10.1511/1999.30.336. S2CID 221585816.
- Shibata, Takanori; Morioka, Hiroshi; Hayase, Tadakatsu; et al. (17 January 1996). "Highly Enantioselective Catalytic Asymmetric Automultiplication of Chiral Pyrimidyl Alcohol". Journal of the American Chemical Society. 118 (2): 471–472. Bibcode:1996JAChS.118..471S. doi:10.1021/ja953066g.
- Soai, Kenso; Sato, Itaru; Shibata, Takanori (2001). "Asymmetric autocatalysis and the origin of chiral homogeneity in organic compounds". The Chemical Record. 1 (4): 321–332. doi:10.1002/tcr.1017. PMID 11893072.
- Hazen 2005, p. 184
- Meierhenrich, Uwe (2008). Amino acids and the asymmetry of life caught in the act of formation. Berlin: Springer. pp. 76–79. ISBN 978-3-540-76886-9.
- Mullen, Leslie (5 September 2005). "Building Life from Star-Stuff". Astrobiology Magazine. Archived from the original on 14 July 2015.
- Root-Bernstein, Robert (23 June 2010). "Experimental Test of L- and D-Amino Acid Binding to L- and D-Codons Suggests that Homochirality and Codon Directionality Emerged with the Genetic Code". Symmetry. 2 (2): 1180–1200. Bibcode:2010Symm....2.1180R. doi:10.3390/sym2021180.
Sources
- Altermann, Wladyslaw (2009). "Introduction: A Roadmap to Fata Morgana?". In Seckbach, Joseph; Walsh, Maud (eds.). From Fossils to Astrobiology: Records of Life on Earth and the Search for Extraterrestrial Biosignatures. Cellular Origin, Life in Extreme Habitats and Astrobiology. Vol. 12. Dordrecht, the Netherlands; London: Springer. ISBN 978-1-4020-8836-0.
- Bada, Jeffrey L.; Lazcano, Antonio (2009). "The Origin of Life". In Ruse, Michael; Travis, Joseph (eds.). Evolution: The First Four Billion Years. Foreword by Edward O. Wilson. Cambridge: Belknap Press of Harvard University Press. ISBN 978-0-674-03175-3. OCLC 225874308.
- Barton, Nicholas H.; Briggs, Derek E.G.; Eisen, Jonathan A.; et al. (2007). Evolution. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press. ISBN 978-0-87969-684-9. OCLC 86090399.
- Bastian, H. Charlton (1871). The Modes of Origin of Lowest Organisms. London; New York: Macmillan and Company. OCLC 42959303. Retrieved 6 June 2015.
- Bernal, J. D. (1951). The Physical Basis of Life. London: Routledge & Kegan Paul.
- Bernal, J. D. (1960). "The Problem of Stages in Biopoesis". In Florkin, M. (ed.). Aspects of the Origin of Life. International Series of Monographs on Pure and Applied Biology. Oxford, UK; New York: Pergamon Press. ISBN 978-1-4831-3587-8.
- Bernal, J. D. (1967) . The Origin of Life. The Weidenfeld and Nicolson Natural History. Translation of Oparin by Ann Synge. London: Weidenfeld & Nicolson.
- Bock, Gregory R.; Goode, Jamie A., eds. (1996). Evolution of Hydrothermal Ecosystems on Earth (and Mars?). Ciba Foundation Symposium. Vol. 202. Chichester, UK; New York: John Wiley & Sons. ISBN 978-0-471-96509-1.
- Bondeson, Jan (1999). The Feejee Mermaid and Other Essays in Natural and Unnatural History. Ithaca, NY: Cornell University Press. ISBN 978-0-8014-3609-3.
- Bryson, Bill (2004). A Short History of Nearly Everything. London: Black Swan. ISBN 978-0-552-99704-1. OCLC 55589795.
- Calvin, Melvin (1969). Chemical Evolution: Molecular Evolution Towards the Origin of Living Systems on the Earth and Elsewhere. Oxford, UK: Clarendon Press. ISBN 978-0-19-855342-7. OCLC 25220.
- Chaichian, Masud; Rojas, Hugo Perez; Tureanu, Anca (2014). "Physics and Life". Basic Concepts in Physics. Undergraduate Lecture Notes in Physics. Berlin; Heidelberg: Springer Berlin Heidelberg. pp. 353–364. doi:10.1007/978-3-642-19598-3_12. ISBN 978-3-642-19597-6. OCLC 900189038. S2CID 115247432.
- Chang, Thomas Ming Swi (2007). Artificial Cells: Biotechnology, Nanomedicine, Regenerative Medicine, Blood Substitutes, Bioencapsulation, and Cell/Stem Cell Therapy. Regenerative Medicine, Artificial Cells and Nanomedicine. Vol. 1. Hackensack, New Jersey: World Scientific. ISBN 978-981-270-576-1. OCLC 173522612.
- Dalrymple, G. Brent (2001). "The age of the Earth in the twentieth century: a problem (mostly) solved". In Lewis, C.L.E.; Knell, S.J. (eds.). The Age of the Earth: from 4004 BC to AD 2002. Geological Society Special Publication. Vol. 190. London: Geological Society of London. pp. 205–221. Bibcode:2001GSLSP.190..205D. doi:10.1144/gsl.sp.2001.190.01.14. ISBN 978-1-86239-093-5. OCLC 48570033. S2CID 130092094.
- Darwin, Charles (1887). Darwin, Francis (ed.). The Life and Letters of Charles Darwin, Including an Autobiographical Chapter. Vol. 3 (3rd ed.). London: John Murray. OCLC 834491774.
- Davies, Paul (1999). The Fifth Miracle: The Search for the Origin of Life. London: Penguin Books. ISBN 978-0-14-028226-9.
- Dobell, Clifford (1960) . Antony van Leeuwenhoek and His 'Little Animals'. New York: Dover Publications.
- Dyson, Freeman (1999). Origins of Life (Revised ed.). Cambridge, UK; New York: Cambridge University Press. ISBN 978-0-521-62668-2.
- Fesenkov, V. G. (1959). "Some Considerations about the Primaeval State of the Earth". In Oparin, A. I.; et al. (eds.). The Origin of Life on the Earth. I.U.B. Symposium Series. Vol. 1. Edited for the International Union of Biochemistry by Frank Clark and R.L.M. Synge (English-French-German ed.). London; New York: Pergamon Press. ISBN 978-1-4832-2240-0. International Symposium on the Origin of Life on the Earth (held at Moscow, 19–24 August 1957)
- Hazen, Robert M. (2005). Genesis: The Scientific Quest for Life's Origin. Washington, DC: Joseph Henry Press. ISBN 978-0-309-09432-0. OCLC 60321860.
- Huxley, Thomas Henry (1968) . "VIII Biogenesis and Abiogenesis [1870]". Discourses, Biological and Geological. Collected Essays. Vol. VIII (Reprint ed.). New York: Greenwood Press. OCLC 476737627. Archived from the original on 7 May 2015. Retrieved 19 May 2014.
- Klyce, Brig (22 January 2001). Kingsley, Stuart A.; Bhathal, Ragbir (eds.). Panspermia Asks New Questions. The Search for Extraterrestrial Intelligence (SETI) in the Optical Spectrum III. Vol. 4273. Bellingham, WA: SPIE. doi:10.1117/12.435366. ISBN 0-8194-3951-7. Archived from the original on 3 September 2013. Retrieved 9 June 2015. Proceedings of the SPIE held at San Jose, California, 22–24 January 2001
- Lane, Nick (2009). Life Ascending: The 10 Great Inventions of Evolution (1st American ed.). New York: W.W. Norton & Company. ISBN 978-0-393-06596-1. OCLC 286488326.
- Lennox, James G. (2001). Aristotle's Philosophy of Biology: Studies in the Origins of Life Science. Cambridge Studies in Philosophy and Biology. Cambridge, UK; New York: Cambridge University Press. ISBN 978-0-521-65976-5.
- Michod, Richard E. (1999). Darwinian Dynamics: Evolutionary Transitions in Fitness and Individuality. Princeton, New Jersey: Princeton University Press. ISBN 978-0-691-02699-2. OCLC 38948118.
- Oparin, A.I. (1953) . The Origin of Life. Translation and new introduction by Sergius Morgulis (2nd ed.). Mineola, NY: Dover Publications. ISBN 978-0-486-49522-4.
- Ross, Alexander (1652). Arcana Microcosmi. Vol. II. London. OCLC 614453394. Archived from the original on 24 February 2024. Retrieved 14 July 2015.
- Sheldon, Robert B. (22 September 2005). "Historical Development of the Distinction between Bio- and Abiogenesis" (PDF). In Hoover, Richard B.; Levin, Gilbert V.; Rozanov, Alexei Y.; Gladstone, G. Randall (eds.). Astrobiology and Planetary Missions. Astrobiology and Planetary Missions. Vol. 5906. Bellingham, WA: SPIE. pp. 59061I. doi:10.1117/12.663480. ISBN 978-0-8194-5911-4. Archived (PDF) from the original on 13 April 2015. Retrieved 13 April 2015. Proceedings of the SPIE held at San Diego, California, 31 July–2 August 2005
- Tyndall, John (1905) . Fragments of Science. Vol. 2 (6th ed.). New York: P.F. Collier & Sons. OCLC 726998155.
- Voet, Donald; Voet, Judith G. (2004). Biochemistry. Vol. 1 (3rd ed.). New York: John Wiley & Sons. ISBN 978-0-471-19350-0.
- Yarus, Michael (2010). Life from an RNA World: The Ancestor Within. Cambridge, Massachusetts: Harvard University Press. p. 287. ISBN 978-0-674-05075-4.
External links
Library resources aboutAbiogenesis
- Making headway with the mysteries of life's origins – Adam Mann (PNAS; 14 April 2021)
- Exploring Life's Origins Archived 8 April 2023 at the Wayback Machine a virtual exhibit at the Museum of Science (Boston)
- How life began on Earth – Marcia Malory (Earth Facts; 2015)
- The Origins of Life – Richard Dawkins et al. (BBC Radio; 2004)
- Life in the Universe – Essay by Stephen Hawking (1996)
| Origin of life | |
|---|---|
| History of research |
|
| Prebiotic synthesis | |
| Protocells | |
| Earliest organisms | |
| Research | |
| Elements of nature | |||||
|---|---|---|---|---|---|
| Universe | |||||
| Earth | |||||
| Weather | |||||
| Natural environment | |||||
| Life | |||||
| See also | |||||
