| Revision as of 07:22, 3 August 2011 editLuckas-bot (talk | contribs)929,662 editsm r2.7.1) (robot Adding: tl:Fluoroiodomethane← Previous edit | Latest revision as of 15:55, 31 October 2021 edit undoCipintina (talk | contribs)155 editsm →Synthesis and uses | ||
| (21 intermediate revisions by 18 users not shown) | |||
| Line 1: | Line 1: | ||
| {{chembox | {{chembox | ||
| | Watchedfields = changed | |||
| | verifiedrevid = |
| verifiedrevid = 442806496 | ||
| | ImageFile = Fluoroiodomethane. |
| ImageFile = Fluoroiodomethane Formula V1.svg | ||
| | ImageSize = 120px | | ImageSize = 120px | ||
| | PIN = Fluoro(iodo)methane <!-- Parentheses are used according to Subsection P-16.5.1.3 of Nomenclature of Organic Chemistry – IUPAC Recommendations and Preferred Names 2013 (Blue Book) --> | |||
| | IUPACName = Fluoroiodomethane | |||
| | OtherNames = Fluoro-iodo-methane |
| OtherNames = Fluoroiodomethane<br />Fluoro-iodo-methane<br />Fluoromethyl iodide | ||
| | |
|Section1={{Chembox Identifiers | ||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| | ChemSpiderID = 10329326 | | ChemSpiderID = 10329326 | ||
| Line 12: | Line 13: | ||
| | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChIKey = XGVXNTVBGYLJIR-UHFFFAOYSA-N | | StdInChIKey = XGVXNTVBGYLJIR-UHFFFAOYSA-N | ||
| | CASNo_Ref = {{cascite|correct|??}} | |||
| | CASNo = 373-53-5 | | CASNo = 373-53-5 | ||
| | PubChem = | | PubChem = 13981373 | ||
| | SMILES = FCI | | SMILES = FCI | ||
| | InChI = 1/CH2FI/c2-1-3/h1H2 | | InChI = 1/CH2FI/c2-1-3/h1H2 | ||
| }} | }} | ||
| | |
|Section2={{Chembox Properties | ||
| | Formula = CH<sub>2</sub>FI | | Formula = CH<sub>2</sub>FI | ||
| | MolarMass = 159.93 g/mol | | MolarMass = 159.93 g/mol | ||
| Line 23: | Line 25: | ||
| | Density = | | Density = | ||
| | MeltingPt = | | MeltingPt = | ||
| | |
| BoilingPtC = 53.4 | ||
| | Solubility = | | Solubility = | ||
| }} | }} | ||
| | |
|Section3={{Chembox Hazards | ||
| | MainHazards = | | MainHazards = | ||
| | FlashPt = | | FlashPt = | ||
| | |
| AutoignitionPt = | ||
| | GHSPictograms = {{GHS06}} | |||
| | GHSSignalWord = Danger | |||
| | HPhrases = {{H-phrases|301|311|330}} | |||
| | PPhrases = {{P-phrases|260|264|270|271|280|284|301+310|302+352|304+340|310|312|320|321|322|330|361|363|403+233|405|501}} | |||
| }} | }} | ||
| }} | }} | ||
| '''Fluoroiodomethane''' is |
'''Fluoroiodomethane''' is the ] with the formula FCH<sub>2</sub>I. Also classified as a fluoroiodocarbon (FIC), it is a colorless liquid. It is a ] for the introduction of the fluoromethyl (FCH<sub>2</sub>) group. | ||
| ==Synthesis and uses== | |||
| Fluoroiodomethane can be prepared from ].<ref></ref> | |||
| It is prepared by fluorination of ].<ref>{{cite encyclopedia|chapter=Fluoroiodomethane|last1=Landelle|first1=Gregory|title=Encyclopedia of Reagents for Organic Synthesis|last2=Paquin|first2=Jean-Francois|encyclopedia=e-EROS Encyclopedia of Reagents for Organic Synthesis|year=2011|doi=10.1002/047084289X.rn01273|isbn=978-0471936237}}</ref> | |||
| Its ] fluoroiodomethane |
Its ] fluoroiodomethane is used for fluoromethylation of ]. | ||
| ==Additional reading== | |||
| Also proposal of its use as a safe high-performance foam blowing agent exists.<ref></ref> | |||
| ⚫ | *{{cite journal |author1=Zheng L. |author2=Berridge M. S. | date = January 2000 | title = Synthesis of [<sup>18</sup>F]fluoromethyl iodide, a synthetic precursor for fluoromethylation of radiopharmaceuticals | journal = Applied Radiation and Isotopes | volume = 52 | issue = 1 | pages = 55–61(7) | pmid = 10670923 | doi = 10.1016/S0969-8043(99)00061-5 }} | ||
| ⚫ | *{{cite journal |author1=Chin F. T. |author2=Morse Ch. L. |author3=Shetty H. U. |author4=Pike V. W. | date = December 2005 | title = Automated radiosynthesis of [18F]SPA-RQ for imaging human brain NK1 receptors with PET | journal = Journal of Labelled Compounds and Radiopharmaceuticals | volume = 49 | issue = 1 | pages = 17–31(15) | doi = 10.1002/jlcr.1016 | url = http://www3.interscience.wiley.com/cgi-bin/abstract/112221807/ABSTRACT?CRETRY=1&SRETRY=0 | access-date = 2007-06-29 }}{{dead link|date=February 2019|bot=medic}}{{cbignore|bot=medic}} | ||
| ⚫ | *{{cite journal |author1=Tedder, J. M. |author2=Sloan, J. P. |author3=Walton, J. C. | date = 1975 | title = Free Radical Addition to Olefins, Part XVII. Addition of Fluoroiodomethane to Fluoroethylenes| journal = Journal of the Chemical Society | pages = 1846–1850 }} | ||
| == |
==References== | ||
| {{reflist}} | {{reflist}} | ||
| ==References== | |||
| ⚫ | *{{cite journal | |
||
| ⚫ | *{{cite journal | |
||
| ⚫ | *{{cite journal | |
||
| {{Halomethanes}} | {{Halomethanes}} | ||
| Line 57: | Line 62: | ||
| {{organohalide-stub}} | {{organohalide-stub}} | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 15:55, 31 October 2021
| |
| Names | |
|---|---|
| Preferred IUPAC name Fluoro(iodo)methane | |
| Other names
Fluoroiodomethane Fluoro-iodo-methane Fluoromethyl iodide | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.201.539 |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | CH2FI |
| Molar mass | 159.93 g/mol |
| Boiling point | 53.4 °C (128.1 °F; 326.5 K) |
| Hazards | |
| GHS labelling: | |
| Pictograms | 
|
| Signal word | Danger |
| Hazard statements | H301, H311, H330 |
| Precautionary statements | P260, P264, P270, P271, P280, P284, P301+P310, P302+P352, P304+P340, P310, P312, P320, P321, P322, P330, P361, P363, P403+P233, P405, P501 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
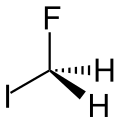
Fluoroiodomethane is the halomethane with the formula FCH2I. Also classified as a fluoroiodocarbon (FIC), it is a colorless liquid. It is a reagent for the introduction of the fluoromethyl (FCH2) group.
Synthesis and uses
It is prepared by fluorination of methylene iodide.
Its isotopomer fluoroiodomethane is used for fluoromethylation of radiopharmaceuticals.
Additional reading
- Zheng L.; Berridge M. S. (January 2000). "Synthesis of [F]fluoromethyl iodide, a synthetic precursor for fluoromethylation of radiopharmaceuticals". Applied Radiation and Isotopes. 52 (1): 55–61(7). doi:10.1016/S0969-8043(99)00061-5. PMID 10670923.
- Chin F. T.; Morse Ch. L.; Shetty H. U.; Pike V. W. (December 2005). "Automated radiosynthesis of [18F]SPA-RQ for imaging human brain NK1 receptors with PET". Journal of Labelled Compounds and Radiopharmaceuticals. 49 (1): 17–31(15). doi:10.1002/jlcr.1016. Retrieved 2007-06-29.
- Tedder, J. M.; Sloan, J. P.; Walton, J. C. (1975). "Free Radical Addition to Olefins, Part XVII. Addition of Fluoroiodomethane to Fluoroethylenes". Journal of the Chemical Society: 1846–1850.
References
- Landelle, Gregory; Paquin, Jean-Francois (2011). "Fluoroiodomethane". Encyclopedia of Reagents for Organic Synthesis. e-EROS Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rn01273. ISBN 978-0471936237.
| Halomethanes | |
|---|---|
| Unsubstituted | |
| Monosubstituted | |
| Disubstituted | |
| Trisubstituted | |
| Tetrasubstituted | |
| * Chiral compound. | |
This article about an organic halide is a stub. You can help Misplaced Pages by expanding it. |