| |
| Names | |
|---|---|
| Preferred IUPAC name Tribromo(fluoro)methane | |
| Other names
Tribromofluoromethane Tribromo-fluoro-methane Fluorotribromomethane Halon 1103 FC-11B3 R 11B3 | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.005.942 |
| EC Number |
|
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
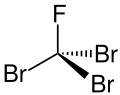
| Chemical formula | CBr3F |
| Molar mass | 270.72 g/mol |
| Appearance | Clear yellow liquid |
| Density |
|
| Melting point | −73 °C (−99 °F; 200 K) |
| Boiling point | 108 °C (226 °F; 381 K) |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
| Main hazards | Irritant |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Tribromofluoromethane or Halon 1103 or R 11B3 is a fully halogenated mixed halomethane or, more exactly, a bromofluorocarbon (BFC). It is a colorless to yellow liquid.
Tribromofluoromethane can be used in fire extinguishers.
Table of physical properties
| Property | Value |
|---|---|
| Refractive index, n, at 20 °C | 1.5216 |
| Surface tension at 20 °C | 31.68 mN·m |
| Viscosity at 0 °C | 2.09 mPa·s, 2.09 cP |
External links
References
- PubChem. "Tribromofluoromethane". pubchem.ncbi.nlm.nih.gov. Retrieved 2022-11-25.
- "Tribromofluoromethane 98.0 %, TCI America, Quantity: 5g | Fisher Scientific". www.fishersci.com. Retrieved 2022-11-25.
| Halomethanes | |
|---|---|
| Unsubstituted | |
| Monosubstituted | |
| Disubstituted | |
| Trisubstituted | |
| Tetrasubstituted | |
| * Chiral compound. | |
This article about an organic halide is a stub. You can help Misplaced Pages by expanding it. |