| |
| Names | |
|---|---|
| Other names Vanadium(II) fluoride | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| PubChem CID | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | F2V |
| Molar mass | 88.9383 g·mol |
| Appearance | blue crystals
|
| Solubility in water | soluble in water, forms |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Vanadium(II) fluoride is a fluoride of vanadium, with the chemical formula of VF2. It forms blue crystals.
Preparation
Vanadium(II) fluoride can be produced by the reduction of vanadium trifluoride by hydrogen in a hydrogen fluoride atmosphere at 1150 °C:
- 2 VF3 + H2 → 2 VF2 + 2 HF
Properties
Physical properties
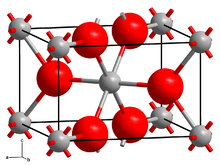
Vanadium(II) fluoride crystallizes in the tetragonal crystal system with space group P42/mnm (No. 136). Its lattice constants are a = 480.4 pm and c = 323.7 pm.
Reactions
Vanadium(II) fluoride is a strong reducing agent that can reduce nitrogen to hydrazine in the presence of magnesium hydroxide.
It dissolves in water to form ions.
- V + 6 H2O → [V(H2O)6]
References
- "WebElements Periodic Table » Vanadium » vanadium difluoride". www.webelements.com. Retrieved 2023-09-16.
- ^ Holleman, Arnold Frederik; Wiberg, Egon (2001), Wiberg, Nils (ed.), Inorganic Chemistry, translated by Eagleson, Mary; Brewer, William, San Diego/Berlin: Academic Press/De Gruyter, p. 1550, ISBN 0-12-352651-5
- Lothar Kolditz: Anorganische Chemie Teil 2. VEB Deutscher Verlag der Wissenschaften, Berlin, 1980, S. 641.
- J. W. Stout, W. O. J. Boo: Crystalline vanadium (II) fluoride, VF2. Preparation, structure, heat capacity from 5 to 300 K and magnetic ordering. In: The Journal of Chemical Physics. 71, 1, 1979, S. 1–8, doi:10.1063/1.438115.
| Vanadium compounds | |||||
|---|---|---|---|---|---|
| Vanadium(0) | |||||
| Vanadium(II) | |||||
| Vanadium(III) |
| ||||
| Vanadium(IV) |
| ||||
| Vanadium(V) |
| ||||