Chemical compound with formula RhF₆
Chemical compound
Rhodium hexafluoride , also rhodium(VI) fluoride , (RhF6 ) is the inorganic compound of rhodium and fluorine . A black volatile solid, it is a highly reactive material which starts to slowly thermally decompose already at room temperature and a rare example of a rhodium(VI) compound. It is one of seventeen known binary hexafluorides .
Rhodium hexafluoride was discovered by American radiochemists in 1961, soon after the discovery of ruthenium hexafluoride . It is prepared by reaction of rhodium metal with an excess of elemental fluorine :
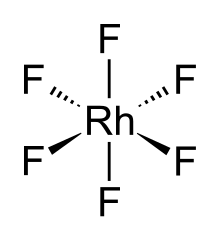
Rh + 3 F2 → RhF6 The RhF6 molecule has octahedral molecular geometry . Consistent with its d configuration, the six Rh–F bond lengths are equivalent, being 1.824 Å. It crystallises in an orthorhombic space group Pnma with lattice parameters of a = 9.323 Å , b = 8.474 Å, and c = 4.910 Å.
Like some other metal fluorides, RhF6 is highly oxidizing. It attacks glass, and can even react with elemental oxygen .
References
^ CRC Handbook of Chemistry and Physics ISBN 978-1-4200-9084-0 , Section 4, Physical Constants of Inorganic Compounds , p. 4-85.
^ Drews, T.; Supeł, J.; Hagenbach, A.; Seppelt, K. (2006). "Solid State Molecular Structures of Transition Metal Hexafluorides". Inorganic Chemistry 45 (9): 3782–3788. doi :10.1021/ic052029f . PMID 16634614 .
Chernick, Cedric L.; Claassen, Howard H.; Weinstock, Bernard (1961). "RHODIUM HEXAFLUORIDE" . Journal of the American Chemical Society . 83 (14): 3165–3166. doi :10.1021/ja01475a046 . ISSN 0002-7863 .
^ 《无机化学丛书》第九卷:锰分族、铁系、铂系 (in Chinese). 北京: 科学出版社. 1991. p. 478. ISBN 7-03-002238-6
Riedel, Sebastian; Kaupp, Martin (2009). "The highest oxidation states of the transition metal elements" (PDF). Coordination Chemistry Reviews . 253 (5–6). Elsevier: 606–624. doi :10.1016/j.ccr.2008.07.014 .
Further reading
External links
Binary hexafluorides Known binary hexafluorides Predicted binary hexafluorides Noble gas binary hexafluorides
Transition metal binary hexafluorides
Actinide binary hexafluorides
Rhodium compounds Rh(0) Rh(I)
Rh(II)
Organorhodium(II) compunds
Rh(III)
Rh(IV)
Rh(V)
Rh(VI)
Fluorine compounds Salts and covalent derivatives of the fluoride ion
PF−6, AsF−6, SbF−6 compounds
AlF2−5, AlF3−6 compounds
chlorides, bromides, iodides
SiF2−6, GeF2−6 compounds
Oxyfluorides
Organofluorides
with transition metal,
nitric acids
bifluorides
thionyl, phosphoryl,
Chemical formulas
Salts and covalent derivatives of the fluoride ion
Categories :
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.
**DISCLAIMER** We are not affiliated with Wikipedia, and Cloudflare.
The information presented on this site is for general informational purposes only and does not constitute medical advice.
You should always have a personal consultation with a healthcare professional before making changes to your diet, medication, or exercise routine.
AI helps with the correspondence in our chat.
We participate in an affiliate program. If you buy something through a link, we may earn a commission 💕
↑
 Media related to Rhodium hexafluoride at Wikimedia Commons
Media related to Rhodium hexafluoride at Wikimedia Commons