| |||

| |||
| Identifiers | |||
|---|---|---|---|
| CAS Number | |||
| 3D model (JSmol) | |||
| ChemSpider | |||
| ECHA InfoCard | 100.033.734 | ||
| PubChem CID | |||
| RTECS number |
| ||
| UNII | |||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | ClF5 | ||
| Molar mass | 130.445 g mol | ||
| Appearance | colorless gas | ||
| Density | 4.5 kg/m (g/L) | ||
| Melting point | −103 °C (−153 °F; 170 K) | ||
| Boiling point | −13.1 °C (8.4 °F; 260.0 K) | ||
| Solubility in water | Hydrolyzes | ||
| Structure | |||
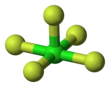
| Molecular shape | Square pyramidal | ||
| Thermochemistry | |||
| Std molar entropy (S298) |
310.73 J K mol | ||
| Std enthalpy of formation (ΔfH298) |
−238.49 kJ mol | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Chlorine pentafluoride is an interhalogen compound with formula ClF5. This colourless gas is a strong oxidant that was once a candidate oxidizer for rockets. The molecule adopts a square pyramidal structure with C4v symmetry, as confirmed by its high-resolution F NMR spectrum. It was first synthesized in 1963.
Preparation
Some of the earliest research on the preparation was classified. It was first prepared by fluorination of chlorine trifluoride at high temperatures and high pressures:
- ClF3 + F2 → ClF5
- ClF + 2F2 → ClF5
- Cl2 + 5F2 → 2ClF5
- CsClF4 + F2 → CsF + ClF5
NiF2 catalyzes this reaction.
Certain metal fluorides, MClF4 (i.e. KClF4, RbClF4, CsClF4), react with F2 to produce ClF5 and the corresponding alkali metal fluoride.
Reactions
In a highly exothermic reaction, ClF5 reacts with water to produce chloryl fluoride and hydrogen fluoride:
- ClF
5 + 2 H
2O → ClO
2F + 4 HF
It is also a strong fluorinating agent. At room temperature it reacts readily with all elements (including otherwise "inert" elements like platinum and gold) except noble gases, nitrogen, oxygen and fluorine.
Uses
Rocket propellant
Chlorine pentafluoride was once considered for use as an oxidizer for rockets. As a propellant, it has a higher maximum specific impulse than ClF3, but with the same difficulties in handling. Due to the hazardous nature of chlorine pentafluoride, it has yet to be used in a large scale rocket propulsion system.
See also
References
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 833. ISBN 978-0-08-037941-8.
- ^ Pilipovich, D.; Maya, W.; Lawton, E.A.; Bauer, H.F.; Sheehan, D. F.; Ogimachi, N. N.; Wilson, R. D.; Gunderloy, F. C.; Bedwell, V. E. (1967). "Chlorine pentafluoride. Preparation and Properties". Inorganic Chemistry. 6 (10): 1918. doi:10.1021/ic50056a036.
- Smith D. F. (1963). "Chlorine Pentafluoride". Science. 141 (3585): 1039–1040. Bibcode:1963Sci...141.1039S. doi:10.1126/science.141.3585.1039. PMID 17739492. S2CID 39767609.
- ^ Clark, John Drury (23 May 2018). Ignition!: An Informal History of Liquid Rocket Propellants. Rutgers University Press. pp. 87–88. ISBN 978-0-8135-9918-2.
- ^ Smith D. F. (1963). "Chlorine Pentafluoride". Science. 141 (3585): 1039–1040. Bibcode:1963Sci...141.1039S. doi:10.1126/science.141.3585.1039. PMID 17739492. S2CID 39767609.
- Šmalc A, Žemva B, Slivnik J, Lutar K (1981). "On the Synthesis of Chlorine Pentafluoride". Journal of Fluorine Chemistry. 17 (4): 381–383. doi:10.1016/S0022-1139(00)81783-2.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 834. ISBN 978-0-08-037941-8.
External links
- National Pollutant Inventory - Fluoride and compounds fact sheet
- New Jersey Hazardous Substance Fact Sheet
- WebBook page for ClF5
| Chlorine compounds | |
|---|---|
| Chlorides and acids | |
| Chlorine fluorides | |
| Chlorine oxides | |
| Chlorine oxyfluorides | |
| Chlorine(I) derivatives | |