| |
| Names | |
|---|---|
| Other names Vanadium oxyfluoride, trifluorooxovanadium | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.033.849 |
| PubChem CID | |
SMILES
| |
| Properties | |
| Chemical formula | F3OV |
| Molar mass | 123.9599 g/mol |
| Appearance | yellowish orange powder |
| Density | 2.4590 g/cm |
| Melting point | 300 °C (572 °F; 573 K) |
| Boiling point | 480 °C (896 °F; 753 K) |
| Solubility in water | insoluble |
| Hazards | |
| GHS labelling: | |
| Pictograms |   
|
| Signal word | Danger |
| Hazard statements | H302, H312, H314, H332 |
| Precautionary statements | P260, P261, P264, P270, P271, P280, P301+P310, P301+P312, P301+P330+P331, P302+P352, P303+P361+P353, P304+P312, P304+P340, P305+P351+P338, P310, P312, P322, P330, P361, P363, P405, P501 |
| NFPA 704 (fire diamond) |
 |
| Related compounds | |
| Related compounds | VF5 VOCl3 VO2F |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
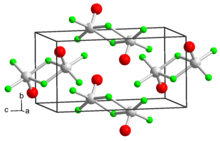
Vanadium(V) oxytrifluoride is a chemical compound with the formula VOF3. It is one of several vanadium(V) oxyhalides. VOF3 is a yellowish orange powder that is sensitive to moisture. Characteristic of early metal fluorides, the structure is polymeric in the solid state. The solid adopts a layered structure but upon evaporation, the species becomes dimeric. In contrast VOCl3 and VOBr3 remain tetrahedral in all states, being volatile liquids at room temperature.
Reactions
In organic synthesis, VOF3 is used for the oxidative coupling of phenols, for example in the syntheses of vancomycin and its analogues. For these applications VOF3 is typically dissolved in trifluoroacetic acid.
Vanadium(V) oxytrifluoride reacts with hexamethyldisiloxane to give vanadium dioxide fluoride:
- (CH3)3SiOSi(CH3)3 + VOF3 → VO2F + 2 (CH3)3SiF
References
- "Trifluorooxovanadium". pubchem.ncbi.nlm.nih.gov.
- Perry, Dale L. (2011). Handbook of Inorganic Compounds. CRC Press. ISBN 978-1-4398-1461-1.
- Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- Vanasse, Benoit; O'Brien, Michael K. (2001). "Vanadyl Trifluoride". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rv005. ISBN 0471936235.
- Davis, Martin F.; Jura, Marek; Leung, Alethea; Levason, William; Littlefield, Benjamin; Reid, Gillian; Webster, Michael (2008). "Synthesis, Chemistry and Structures of Complexes of the Dioxovanadium(v) Halides VO2F and VO2Cl". Dalton Transactions (44): 6265–6273. doi:10.1039/b811422f. PMID 18985260.
| Vanadium compounds | |||||
|---|---|---|---|---|---|
| Vanadium(0) | |||||
| Vanadium(II) | |||||
| Vanadium(III) |
| ||||
| Vanadium(IV) |
| ||||
| Vanadium(V) |
| ||||
This inorganic compound–related article is a stub. You can help Misplaced Pages by expanding it. |