 Ra 0 F | |
| Identifiers | |
|---|---|
| CAS Number | |
| 3D model (JSmol) | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | RaF2 |
| Molar mass | 263.8214 g/mol |
| Appearance | White cubic crystals |
| Density | 6.7 g/cm |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
| Main hazards | Highly radioactive and toxic |
| GHS labelling: | |
| Pictograms | 
|
| Hazard statements | H350 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
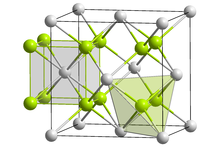
Radium fluoride is an inorganic compound with a chemical formula of RaF2. This salt, like all radium compounds, is highly radioactive. It can be coprecipitated with lanthanide fluorides. Radium fluoride has the same crystal form as calcium fluoride (fluorite). However, calculations suggest that radium fluoride vapor consists of RaF2 molecules, with a bond angle of 118°, due to substantial covalent interaction within the molecule.
Production
Radium fluoride can be produced by the reaction of radium metal and hydrogen fluoride gas:
- Ra + 2 HF → RaF2 + H2
References
- ^ "Radium fluoride | 20610-49-5".
- US 1655184, Hahn, Otto, "Radium preparation and process of making same", published 1928-01-03
- Lee, Edmond P. F.; Soldán, Pavel; Wright, Timothy G. (2001-11-01). "The Heaviest Group 2 Difluoride, RaF 2 : Geometry and Ionization Energy". Inorganic Chemistry. 40 (23): 5979–5984. doi:10.1021/ic010538l. ISSN 0020-1669.
See also
- Monica Vasiliu, J. Grant Hill, Kirk A. Peterson, David A. Dixon (2018-01-11). "Structures and Heats of Formation of Simple Alkaline Earth Metal Compounds II: Fluorides, Chlorides, Oxides, and Hydroxides for Ba, Sr, and Ra" (PDF). The Journal of Physical Chemistry A. 122 (1): 316–327. Bibcode:2018JPCA..122..316V. doi:10.1021/acs.jpca.7b09056. ISSN 1089-5639. PMID 29240428.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
| Radium compounds | |
|---|---|