Chemical compound
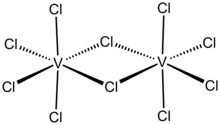
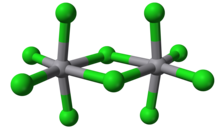
Vanadium(V) chloride is the inorganic compound with the formula VCl5 . It is a black diamagnetic solid. The molecules adopt a bioctahedral structure similar to that of niobium(V) chloride .
Preparation and reactions
Chlorine cannot oxidise vanadium(IV); chlorination of vanadium metal will yield only vanadium(IV) chloride . Vanadium(V) chloride is instead prepared from vanadium pentafluoride with excess boron trichloride as a chlorinating agent:
2 VF5 + 10 BCl3 → [VCl5 ]2 + 10 BF2 Cl It is unstable at room temperature with respect to vanadium(IV) chloride , which releases gaseous chlorine :
[VCl5 ]2 → 2 VCl4 + Cl2 In contrast, the heavier analogues NbCl5 and TaCl5 are stable and not particularly oxidizing.
References
Tamadon, Farhad; Seppelt, K. (2012). "The Elusive Halides VCl5 , MoCl6 , and ReCl6 ". Angewandte Chemie International Edition . 52 (2): 767–769. doi :10.1002/anie.201207552 . PMID 23172658 .
Salts and covalent derivatives of the chloride ion
Categories :
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.
**DISCLAIMER** We are not affiliated with Wikipedia, and Cloudflare.
The information presented on this site is for general informational purposes only and does not constitute medical advice.
You should always have a personal consultation with a healthcare professional before making changes to your diet, medication, or exercise routine.
AI helps with the correspondence in our chat.
We participate in an affiliate program. If you buy something through a link, we may earn a commission 💕
↑