| Names | |
|---|---|
| Other names Neodymium dichloride | |
| Identifiers | |
| 3D model (JSmol) | |
| ChemSpider | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | NdCl2 |
| Molar mass | 215.14 g/mol |
| Appearance | Black solid |
| Structure | |
| Crystal structure | Orthorhombic |
| Space group | Pnma, No. 62 |
| Related compounds | |
| Other anions | Neodymium(II) bromide Neodymium(II) iodide |
| Other cations | SmCl2, EuCl2, DyCl2, TmCl2, YbCl2, |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Neodymium(II) chloride or neodymium dichloride is a chemical compound of neodymium and chlorine with the formula NdCl2.
Preparation
Neodymium(II) chloride can be prepared by reducing neodymium(III) chloride with lithium metal/naphthalene or lithium chloride in THF.
Reduction of neodymium(III) chloride with neodymium metal at temperatures above 650 °C also yields neodymium(II) chloride:
- 2 NdCl3 + Nd → 3 NdCl2
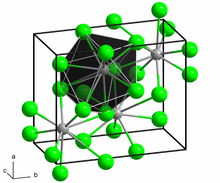
Structure
Neodymium(II) chloride adopts the PbCl2 (cotunnite) structure. Each Nd ion is coordinated by nine Cl ions in a tricapped trigonal prismatic arrangement. Seven of the Nd-Cl distances are in the range 2.95-3.14 Å while two are longer at 3.45 Å.
References
- Brauer, Georg; Baudler, Marianne (1975). Handbuch der Präparativen Anorganischen Chemie, Band I. (3rd ed.). Stuttgart: Ferdinand Enke. ISBN 3-432-02328-6.
- Rossmainth, K. (1979-07-01). "Herstellung von Neodym(II)-chlorid in Lösung" [Preparation of neodymium(II) chloride in solution]. Monatshefte für Chemie - Chemical Monthly. 110 (4): 1019–1023. doi:10.1007/BF00906697. S2CID 99130833.
- Gerd Meyer, Lester R. Morss (1991). Synthesis of lanthanide and actinide compounds. Springer. p. 161. ISBN 0-7923-1018-7.
- Meyer, Gerd; Schleid, Thomas (1985). "Zweiwertiges Neodym: NdCl2 und KNd2Cl5". Z. anorg. allg. Chem. 528 (9): 55–60. doi:10.1002/zaac.19855280906.
- "ICSD Entry: 48206". Cambridge Structural Database: Access Structures. Cambridge Crystallographic Data Centre. Retrieved 2021-06-06.
| Neodymium compounds | |
|---|---|
| Nd(II) | |
| Nd(III) | |
| Nd(IV) | |
| Lanthanide salts of halides | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
This inorganic compound–related article is a stub. You can help Misplaced Pages by expanding it. |