 Anhydrous | |
 Tetrahydrate | |
| |
| Names | |
|---|---|
| Other names
Indium chloride Indium trichloride | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.030.027 |
| EC Number |
|
| PubChem CID | |
| RTECS number |
|
| UNII | |
| UN number | 3260 |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | InCl3 |
| Molar mass | 221.18 g/mol |
| Appearance | white flakes |
| Density | 3.46 g/cm |
| Melting point | 586 °C (1,087 °F; 859 K) |
| Boiling point | 800 °C (1,470 °F; 1,070 K) |
| Solubility in water | 195 g/100 mL, exothermic |
| Solubility in other solvents | THF, Ethanol |
| Structure | |
| Crystal structure | Monoclinic, mS16 |
| Space group | C12/m1, No. 12 |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
| Main hazards | Corrosive |
| GHS labelling: | |
| Pictograms |  
|
| Signal word | Danger |
| Hazard statements | H302, H314 |
| Precautionary statements | P260, P301+P330+P331, P303+P361+P353, P305+P351+P338, P405, P501 |
| NFPA 704 (fire diamond) |
 |
| Safety data sheet (SDS) | External SDS |
| Related compounds | |
| Other anions | Indium(III) fluoride Indium(III) bromide Indium(III) iodide |
| Other cations | Aluminium chloride Gallium trichloride Thallium(III) chloride |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Indium(III) chloride is the chemical compound with the formula InCl3 which forms a tetrahydrate. This salt is a white, flaky solid with applications in organic synthesis as a Lewis acid. It is also the most available soluble derivative of indium. This is one of three known indium chlorides.
Synthesis and structure
Being a relatively electropositive metal, indium reacts quickly with chlorine to give the trichloride. Indium trichloride is very soluble and deliquescent. A synthesis has been reported using an electrochemical cell in a mixed methanol-benzene solution.
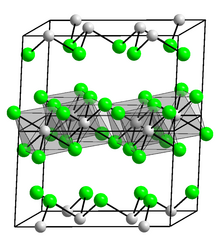
Like AlCl3 and TlCl3, InCl3 crystallizes as a layered structure consisting of a close-packed chloride arrangement containing layers of octahedrally coordinated In(III) centers, a structure akin to that seen in YCl3. In contrast, GaCl3 crystallizes as dimers containing Ga2Cl6. Molten InCl3 conducts electricity, whereas AlCl3 does not as it converts to the molecular dimer, Al2Cl6.
Reactions
InCl3 is a Lewis acid and forms complexes with donor ligands, L, InCl3L, InCl3L2, InCl3L3. For example, with the chloride ion it forms tetrahedral InCl4, trigonal bipyramidal InCl5, and octahedral InCl6.
In diethyl ether solution, InCl3 reacts with lithium hydride, LiH, to form . This unstable compound decomposes below 0 °C, and is reacted in situ in organic synthesis as a reducing agent and to prepare tertiary amine and phosphine complexes of InH3.
Trimethylindium, InMe3, can be produced by reacting InCl3 in diethyl ether solution either with the Grignard reagent or methyllithium, LiMe. Triethylindium can be prepared in a similar fashion but with the grignard reagent EtMgBr.
InCl3 reacts with indium metal at high temperature to form the lower valent indium chlorides In5Cl9, In2Cl3 and InCl.
Catalyst in chemistry
Indium chloride is a Lewis acid catalyst in organic reactions such as Friedel-Crafts acylations and Diels-Alder reactions. As an example of the latter, the reaction proceeds at room temperature, with 1 mole% catalyst loading in an acetonitrile-water solvent mixture. The first step is a Knoevenagel condensation between the barbituric acid and the aldehyde; the second step is a reverse electron-demand Diels-Alder reaction, which is a multicomponent reaction of N,N'-dimethyl-barbituric acid, benzaldehyde and ethyl vinyl ether. With the catalyst, the reported chemical yield is 90% and the percentage trans isomer is 70%. Without the catalyst added, the yield drops to 65% with 50% trans product.
References
- ^ "Indium(III) Chloride". American Elements. Retrieved May 15, 2019.
- Araki, S.; Hirashita, T. "Indium trichloride" in Encyclopedia of Reagents for Organic Synthesis (Ed: L. Paquette) 2004, J. Wiley & Sons, New York. doi:10.1002/047084289X.
- Indium Trichloride
- Habeeb, J. J.; Tuck, D. G. "Electrochemical Synthesis of Indium(III) Complexes" Inorganic Syntheses, 1979, volume XIX, ISBN 0-471-04542-X
- ^ Egon Wiberg, Arnold Frederick Holleman (2001) Inorganic Chemistry, Elsevier ISBN 0123526515
- ^ Wells, A.F. Structural Inorganic Chemistry, Oxford: Clarendon Press, 1984. ISBN 0-19-855370-6.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- Anthony John Downs (1993). Chemistry of aluminium, gallium, indium, and thallium. Springer. ISBN 0-7514-0103-X.
- Main Group Metals in Organic Synthesis vol 1, ed. Hisashi Yamamoto, Koichiro Oshima, Wiley VCH, 2004, ISBN 3527305084
- The Group 13 Metals Aluminium, Gallium, Indium and Thallium: Chemical Patterns and Peculiarities, Simon Aldridge, Anthony J. Downs, Wiley, 2011, ISBN 978-0-470-68191-6
- Main Group compounds in Inorganic Syntheses, vol 31, By Schultz, Neumayer, Marks; Ed., Alan H. Cowley, John Wiley & Sons, Inc., 1997, ISBN 0471152889
- An efficient synthesis of novel pyrano- and furopyranopyrimidines via Indium-Catalyzed Multicomponent Domino Reaction Prajapati, D. Mukut Gohain, M. Beilstein Journal of Organic Chemistry 2006, 2:11 doi:10.1186/1860-5397-2-11
| Indium compounds | |||
|---|---|---|---|
| Indium(I) |
| ||
| Indium(I,III) | |||
| Indium(III) |
| ||
 . This unstable compound decomposes below 0 °C, and is reacted in situ in organic synthesis as a reducing agent and to prepare tertiary amine and phosphine complexes of
. This unstable compound decomposes below 0 °C, and is reacted in situ in organic synthesis as a reducing agent and to prepare tertiary amine and phosphine complexes of  or
or 


