| Revision as of 14:05, 24 December 2011 editPetergans (talk | contribs)Extended confirmed users13,256 editsNo edit summary← Previous edit | Latest revision as of 15:08, 15 August 2022 edit undoNucleus hydro elemon (talk | contribs)Extended confirmed users3,331 editsm Moving from Category:Oxohalides to Category:Oxyfluorides using Cat-a-lot | ||
| (28 intermediate revisions by 22 users not shown) | |||
| Line 1: | Line 1: | ||
| {{Chembox | {{Chembox | ||
| | Verifiedfields = changed | |||
| ⚫ | | verifiedrevid = |
||
| | Watchedfields = changed | |||
| | ImageFile = | |||
| ⚫ | | verifiedrevid = 428725254 | ||
| ⚫ | | |
||
| | ImageFile = Selenoyl_fluoride_structure.png | |||
| ⚫ | | |
||
| ⚫ | | ImageSize = 150px | ||
| | ImageFile1 = Selenoyl fluoride 3D Space Filling.png | |||
| | ImageSize1 = 150px | |||
| ⚫ | | ImageAlt = | ||
| | IUPACName = | | IUPACName = | ||
| | PIN = | | PIN = | ||
| | OtherNames = | | OtherNames = | ||
| | |
|Section1={{Chembox Identifiers | ||
| | CASNo_Ref = {{cascite|correct|??}} | |||
| | CASNo = 14984-81-7 | |||
| | |
| CASNo = 14984-81-7 | ||
| | ChemSpiderID_Ref = {{chemspidercite|changed|chemspider}} | |||
| ⚫ | | |
||
| | ChemSpiderID = 10329076 | |||
| ⚫ | | |
||
| | PubChem = 23236013 | |||
| ⚫ | | |
||
| ⚫ | | SMILES = O=(=O)(F)F | ||
| ⚫ | | |
||
| | InChI = 1/F2O2Se/c1-5(2,3)4 | |||
| ⚫ | | |
||
| | InChIKey = VCPFQVXWYPMUKZ-UHFFFAOYAS | |||
| ⚫ | | |
||
| | StdInChI_Ref = {{stdinchicite|changed|chemspider}} | |||
| ⚫ | | |
||
| | StdInChI = 1S/F2O2Se/c1-5(2,3)4 | |||
| ⚫ | | |
||
| | StdInChIKey_Ref = {{stdinchicite|changed|chemspider}} | |||
| ⚫ | | |
||
| | StdInChIKey = VCPFQVXWYPMUKZ-UHFFFAOYSA-N }} | |||
| ⚫ | | |
||
| ⚫ | |Section2={{Chembox Properties | ||
| ⚫ | | |
||
| ⚫ | | Formula = SeO<sub>2</sub>F<sub>2</sub> | ||
| ⚫ | | |
||
| ⚫ | | MolarMass = 148.95 g/mol | ||
| | Autoignition = }} | |||
| ⚫ | | Appearance = Gas. | ||
| ⚫ | | Density = | ||
| | MeltingPtC = -99.5 | |||
| ⚫ | | MeltingPt_ref = <ref>CRC Handbook of Chemistry and Physics</ref> | ||
| | BoilingPtC = -8.4 | |||
| ⚫ | | BoilingPt_ref = <ref>]. "Selenoyl difluoride" ''Inorganic Syntheses'', 1980, volume XX, pp. 36-38. {{ISBN|0-471-07715-1}}.</ref> | ||
| ⚫ | | Solubility = }} | ||
| ⚫ | |Section3={{Chembox Hazards | ||
| ⚫ | | MainHazards = | ||
| ⚫ | | FlashPt = | ||
| | AutoignitionPt = | |||
| }} | |||
| |Section8={{Chembox Related | |||
| | OtherCations = ] | |||
| | OtherCompounds = ],<br />] | |||
| }} | |||
| }} | }} | ||
| '''Selenoyl fluoride''', '''selenoyl difluoride''', '''selenium oxyfluoride''', or '''selenium dioxydifluoride''' is a ] with the ] SeO<sub>2</sub>F<sub>2</sub>. | '''Selenoyl fluoride''', '''selenoyl difluoride''', '''selenium oxyfluoride''', or '''selenium dioxydifluoride''' is a ] with the ] SeO<sub>2</sub>F<sub>2</sub>. | ||
| Line 28: | Line 48: | ||
| ==Structure== | ==Structure== | ||
| The shape of the molecule is a distorted tetrahedron with the O-Se-O angle being 126.2°, the O-Se-F angle being 108.0° and F-Se-F being 94.1°.<ref>Wai-Kee Li, Gong-du Zhou, Thomas C. W. Mak page 651 2008 ISBN |
The shape of the molecule is a distorted tetrahedron with the O-Se-O angle being 126.2°, the O-Se-F angle being 108.0° and F-Se-F being 94.1°.<ref>Wai-Kee Li, Gong-du Zhou, Thomas C. W. Mak page 651 2008 {{ISBN|0-19-921694-0}}</ref> The Se-F bond length is 1.685 Å and the selenium to oxygen bond is 1.575 Å long.<ref>Kolbjørn Hagen, Virginia R. Cross and Kenneth Hedberg "The molecular structure of selenonyl fluoride, SeO<sub>2</sub>F<sub>2</sub>, and sulfuryl fluoride, SO<sub>2</sub>F<sub>2</sub>, as determined by gas-phase electron diffraction" ''Journal of Molecular Structure'' 1978 volume 44 issue 2 page 187 {{doi|10.1016/0022-2860(78)87027-6}}</ref> | ||
| ==Formation== | ==Formation== | ||
| Line 34: | Line 54: | ||
| ==Reactions== | ==Reactions== | ||
| Selenoyl fluoride is more reactive than its analogon ]. It is easier to hydrolyse and to reduce. It may react violently upon contact with ]. | |||
| Selenoyl fluoride reacting with ] gives FXeOSeF<sub>5</sub>.<ref>http://www.scribd.com/doc/30122309/Noble-Gas-Compounds</ref> | |||
| Selenoyl fluoride reacting with ] gives FXeOSeF<sub>5</sub>.<ref>{{cite web |url=https://www.scribd.com/doc/30122309/Noble-Gas-Compounds |title=Noble Gas Compounds |website=www.scribd.com |url-status=dead |archive-url=https://web.archive.org/web/20121104234856/http://www.scribd.com/doc/30122309/Noble-Gas-Compounds |archive-date=2012-11-04}} </ref> | |||
| ==See also== | |||
| *] | |||
| ==References== | ==References== | ||
| Line 44: | Line 63: | ||
| {{Selenium compounds}} | {{Selenium compounds}} | ||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
Latest revision as of 15:08, 15 August 2022
| |

| |
| Identifiers | |
|---|---|
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | SeO2F2 |
| Molar mass | 148.95 g/mol |
| Appearance | Gas. |
| Melting point | −99.5 °C (−147.1 °F; 173.7 K) |
| Boiling point | −8.4 °C (16.9 °F; 264.8 K) |
| Related compounds | |
| Other cations | SO2F2 |
| Related compounds | SeF6, SeO3 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Selenoyl fluoride, selenoyl difluoride, selenium oxyfluoride, or selenium dioxydifluoride is a chemical compound with the formula SeO2F2.
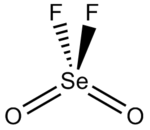
Structure
The shape of the molecule is a distorted tetrahedron with the O-Se-O angle being 126.2°, the O-Se-F angle being 108.0° and F-Se-F being 94.1°. The Se-F bond length is 1.685 Å and the selenium to oxygen bond is 1.575 Å long.
Formation
Selenoyl fluoride can be formed by the action of warm fluorosulfonic acid on barium selenate or selenic acid. SeO3 + SeF4 can give this gas along with other oxyfluorides.
Reactions
Selenoyl fluoride is more reactive than its analogon sulfuryl fluoride. It is easier to hydrolyse and to reduce. It may react violently upon contact with ammonia.
Selenoyl fluoride reacting with xenon difluoride gives FXeOSeF5.
References
- CRC Handbook of Chemistry and Physics
- Seppelt, K. "Selenoyl difluoride" Inorganic Syntheses, 1980, volume XX, pp. 36-38. ISBN 0-471-07715-1.
- Wai-Kee Li, Gong-du Zhou, Thomas C. W. Mak Advanced structural inorganic chemistry page 651 2008 ISBN 0-19-921694-0
- Kolbjørn Hagen, Virginia R. Cross and Kenneth Hedberg "The molecular structure of selenonyl fluoride, SeO2F2, and sulfuryl fluoride, SO2F2, as determined by gas-phase electron diffraction" Journal of Molecular Structure 1978 volume 44 issue 2 page 187 doi:10.1016/0022-2860(78)87027-6
- Advanced Inorganic Chemistry A Comprehensive Text Cotton and Wilkinson
- "Noble Gas Compounds". www.scribd.com. Archived from the original on 2012-11-04.
| Selenium compounds | |
|---|---|
| Se(−II) | |
| Se(0,I) | |
| Se(I) | |
| Se(II) | |
| Se(IV) | |
| Se(VI) | |
| Se(IV,VI) | |