| Revision as of 22:47, 28 October 2023 editKimen8 (talk | contribs)Extended confirmed users5,112 editsm →top: ce← Previous edit | Latest revision as of 06:47, 6 January 2024 edit undoMaxim Masiutin (talk | contribs)Extended confirmed users, IP block exemptions, Pending changes reviewers31,055 edits Add: publisher. Added the cs1 style template to denote Vancouver ("vanc") citation style, because references contain "vauthors" attribute to specify the list of authors. | ||
| Line 1: | Line 1: | ||
| {{Short description|Chemical compound}} | {{Short description|Chemical compound}} | ||
| {{cs1 config|name-list-style=vanc}} | |||
| {{Drugbox | {{Drugbox | ||
| | Verifiedfields = changed | | Verifiedfields = changed | ||
| Line 55: | Line 56: | ||
| }} | }} | ||
| '''Benzethidine''' is a 4-]] derivative that is related to the clinically used ] ] drug ] (''meperidine'', or ''Demerol'').<ref>{{cite book | vauthors = Maul C, Buschmann H, Sundermann B | chapter = Opioids: 3.3 Synthetic Opioids. | title = Analgesics | date = 2005 | pages = 159–169 | isbn = 978-3-527-30403-5 }}</ref> | '''Benzethidine''' is a 4-]] derivative that is related to the clinically used ] ] drug ] (''meperidine'', or ''Demerol'').<ref>{{cite book | vauthors = Maul C, Buschmann H, Sundermann B | chapter = Opioids: 3.3 Synthetic Opioids. | title = Analgesics | date = 2005 | pages = 159–169 | publisher = Wiley-VCH | isbn = 978-3-527-30403-5 }}</ref> | ||
| Benzethidine is not currently used in medicine and is a ]/] drug which is controlled under UN drug conventions. It has similar effects to other opioid derivatives, such as ], ], ] and ].<ref name="pmid13689779">{{cite journal | vauthors = Cahal DA, Dare JG, Keith D | title = A sequential trial of analgesics in labour | journal = The Journal of Obstetrics and Gynaecology of the British Commonwealth | volume = 68 | pages = 88–93 | date = February 1961 | pmid = 13689779 | doi = 10.1111/j.1471-0528.1961.tb02689.x | s2cid = 27397119 }}</ref> In the United States, the drug is a ] with a DEA ACSCN of 9606 and 2014 annual aggregate manufacturing quota of nil.<ref>{{Cite web | url=http://deadiversion.usdoj.gov/quotas/conv_factor/index.html |title = Conversion Factors for Controlled Substances | work = Diversion Control Division | publisher = Drug Enforcement Agency, U.S. Department of Justice }}</ref> The most common salt in use is the hydrochloride, free base conversion ratio of 0.910. | Benzethidine is not currently used in medicine and is a ]/] drug which is controlled under UN drug conventions. It has similar effects to other opioid derivatives, such as ], ], ] and ].<ref name="pmid13689779">{{cite journal | vauthors = Cahal DA, Dare JG, Keith D | title = A sequential trial of analgesics in labour | journal = The Journal of Obstetrics and Gynaecology of the British Commonwealth | volume = 68 | pages = 88–93 | date = February 1961 | pmid = 13689779 | doi = 10.1111/j.1471-0528.1961.tb02689.x | s2cid = 27397119 }}</ref> In the United States, the drug is a ] with a DEA ACSCN of 9606 and 2014 annual aggregate manufacturing quota of nil.<ref>{{Cite web | url=http://deadiversion.usdoj.gov/quotas/conv_factor/index.html |title = Conversion Factors for Controlled Substances | work = Diversion Control Division | publisher = Drug Enforcement Agency, U.S. Department of Justice }}</ref> The most common salt in use is the hydrochloride, free base conversion ratio of 0.910. | ||
Latest revision as of 06:47, 6 January 2024
Chemical compoundPharmaceutical compound
 | |
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
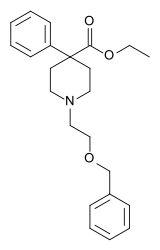
| Formula | C23H29NO3 |
| Molar mass | 367.489 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (what is this?) (verify) | |
Benzethidine is a 4-phenylpiperidine derivative that is related to the clinically used opioid analgesic drug pethidine (meperidine, or Demerol).
Benzethidine is not currently used in medicine and is a Class A/Schedule I drug which is controlled under UN drug conventions. It has similar effects to other opioid derivatives, such as analgesia, sedation, nausea and respiratory depression. In the United States, the drug is a Schedule I Narcotic Controlled Substance with a DEA ACSCN of 9606 and 2014 annual aggregate manufacturing quota of nil. The most common salt in use is the hydrochloride, free base conversion ratio of 0.910.
Legal Status
Australia
Benzethidine is considered a Schedule 9 prohibited substance in Australia under the Poisons Standard (February 2017). A Schedule 9 substance is a substance which may be abused or misused, the manufacture, possession, sale or use of which should be prohibited by law except when required for medical or scientific research, or for analytical, teaching or training purposes with approval of Commonwealth and/or State or Territory Health Authorities.
References
- Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-16.
- Maul C, Buschmann H, Sundermann B (2005). "Opioids: 3.3 Synthetic Opioids.". Analgesics. Wiley-VCH. pp. 159–169. ISBN 978-3-527-30403-5.
- Cahal DA, Dare JG, Keith D (February 1961). "A sequential trial of analgesics in labour". The Journal of Obstetrics and Gynaecology of the British Commonwealth. 68: 88–93. doi:10.1111/j.1471-0528.1961.tb02689.x. PMID 13689779. S2CID 27397119.
- "Conversion Factors for Controlled Substances". Diversion Control Division. Drug Enforcement Agency, U.S. Department of Justice.
- ^ "Poisons Standard". Australian Government. October 2015.
External links
This analgesic-related article is a stub. You can help Misplaced Pages by expanding it. |