| This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed. Find sources: "Acetyldihydrocodeine" – news · newspapers · books · scholar · JSTOR (November 2011) (Learn how and when to remove this message) |
 | |
 | |
| Clinical data | |
|---|---|
| Other names | Acetyldihydrocodeine, Dihydrothebacone, 6-acetyl-7,8-dihydrocodeine |
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.021.253 |
| Chemical and physical data | |
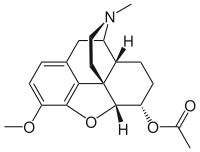
| Formula | C20H25NO4 |
| Molar mass | 343.423 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (what is this?) (verify) | |
Acetyldihydrocodeine is an opiate derivative discovered in Germany in 1914 and was used as a cough suppressant and analgesic. It is not commonly used, but has activity similar to other opiates. Acetyldihydrocodeine is a very close relative derivative of thebacon, where only the 6-7 bond is unsaturated. Acetyldihydrocodeine can be described as the 6-acetyl derivative of dihydrocodeine and is metabolised in the liver by demethylation and deacetylation to produce dihydromorphine.
Since acetyldihydrocodeine has higher lipophilicity than codeine and is converted into dihydromorphine rather than morphine, it can be expected to be more potent and longer-lasting. It also has a higher bioavailability than codeine. Side effects are similar to those of other opiates and include itching, nausea and respiratory depression.
Although an opioid of low to moderate strength and use in medicine elsewhere in the world, acetyldihydrocodeine is a Schedule I controlled substance in the United States. Its DEA Administrative Controlled Substances Control Number is 9051 and the one salt in use, acetyldihydrocodeine hydrochloride, has a freebase conversion ratio of 0.90.
References
- Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-03.
- von Braun J (1914). "Untersuchungen über Morphium-Alkaloide". Chemische Berichte. 47 (2): 2312–2330. doi:10.1002/cber.191404702149.
| Opioid receptor modulators | |||||
|---|---|---|---|---|---|
| μ-opioid (MOR) |
| ||||
| δ-opioid (DOR) |
| ||||
| κ-opioid (KOR) |
| ||||
| Nociceptin (NOP) |
| ||||
| Others |
| ||||
This drug article relating to the respiratory system is a stub. You can help Misplaced Pages by expanding it. |
This analgesic-related article is a stub. You can help Misplaced Pages by expanding it. |