| |
| |
| Names | |
|---|---|
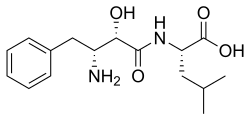
| IUPAC name N--L-leucine | |
| Systematic IUPAC name (2S)-2--4-methylpentanoic acid | |
| Other names Bestatin; N--L-leucine | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.055.917 |
| EC Number |
|
| KEGG | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C16H24N2O4 |
| Molar mass | 308.378 g·mol |
| Melting point | 245 °C (473 °F; 518 K) (decomposes) |
| Hazards | |
| GHS labelling: | |
| Pictograms | 
|
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Ubenimex (INN), also known more commonly as bestatin, is a competitive, reversible protease inhibitor. It is an inhibitor of arginyl aminopeptidase (aminopeptidase B), leukotriene A4 hydrolase (a zinc metalloprotease that displays both epoxide hydrolase and aminopeptidase activities), alanyl aminopeptidase (aminopeptidase M/N), leucyl/cystinyl aminopeptidase (oxytocinase/vasopressinase), and membrane dipeptidase (leukotriene D4 hydrolase). It is being studied for use in the treatment of acute myelocytic leukemia and lymphedema. It is derived from Streptomyces olivoreticuli. Ubenimex has been found to inhibit the enzymatic degradation of oxytocin, vasopressin, enkephalins, and various other peptides and compounds.
See also
References
- N-((2S,3R)-3-Amino-2-hydroxy-4-phenylbutyryl)-L-leucine at Sigma-Aldrich
- "Ubenimex". pubchem.ncbi.nlm.nih.gov. Retrieved 12 December 2021.
- Umezawa, H.; Aoyagi, T.; Suda, H.; Hamada, M.; Takeuchi, T. (1976). "Bestatin, an inhibitor of aminopeptidase B, produced by actinomycetes". The Journal of Antibiotics. 29 (29): 97–99. doi:10.7164/antibiotics.29.97. PMID 931798.
- Muskardin, D.T.; Voelkel, N.F.; Fitzpatrick, F.A. (1994). "Modulation of pulmonary leukotriene formation and perfusion pressure by Bestatin, an inhibitor of leukotriene A4 hydrolase". Biochemical Pharmacology. 48 (48): 131–137. doi:10.1016/0006-2952(94)90232-1. PMID 8043014.
- K Sekine; H Fujii; F Abe (1999). "Induction of apoptosis by Bestatin (ubenimex) in human leukemic cell lines". Leukemia. 13 (5): 729–734. doi:10.1038/sj.leu.2401388. PMID 10374877.
- Nakanishi Y, Nomura S, Okada M, Ito T, Katsumata Y, Kikkawa F, Hattori A, Tsujimoto M, Mizutani S (2000). "Immunoaffinity purification and characterization of native placental leucine aminopeptidase/oxytocinase from human placenta". Placenta. 21 (7): 628–34. doi:10.1053/plac.2000.0564. PMID 10985965.
- Naruki M, Mizutani S, Goto K, Tsujimoto M, Nakazato H, Itakura A, Mizuno K, Kurauchi O, Kikkawa F, Tomoda Y (1996). "Oxytocin is hydrolyzed by an enzyme in human placenta that is identical to the oxytocinase of pregnancy serum". Peptides. 17 (2): 257–61. doi:10.1016/0196-9781(95)02124-8. PMID 8801531. S2CID 28486489.
- Hirayama, Y; Sakamaki, S; Takayanagi, N; Tsuji, Y; Sagawa, T; Chiba, H; Matsunaga, T; Niitsu, Y (2003). "Chemotherapy with ubenimex corresponding to patient age and organ disorder for 18 cases of acute myelogeneous leukemia in elderly patients--effects, complications and long-term survival". Gan to Kagaku Ryoho. Cancer & Chemotherapy. 30 (8): 1113–8. PMID 12938265.
- Tian, W; Rockson, S; Jiang, X; Kim, J; Begaye, A; Shuffle, EM; Tu, AB; Cribb, M; Nepiyushchikh, Z; Feroze, AH; Zamanian, RT; Dhillon, RT; Voelkel, NF; Peters-Golden, M; Kitajewski, J; Dixon, JB; Nicolls, MR (2017). "Leukotriene B4 antagonism ameliorates experimental lymphedema". Science Translational Medicine. 9 (389): eaal3920. doi:10.1126/scitranslmed.aal3920. PMID 28490670.
- Bauvois, B; Dauzonne, D (January 2006). "Aminopeptidase-N/CD13 (EC 3.4.11.2) inhibitors: Chemistry, biological evaluations, and therapeutic prospects". Medicinal Research Reviews. 26 (1): 88–130. doi:10.1002/med.20044. PMC 7168514. PMID 16216010.
External links
| Leukotriene signaling modulators | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Receptor (ligands) |
| ||||||||||||||||
| Enzyme (inhibitors) |
| ||||||||||||||||
| Others | |||||||||||||||||
This pharmacology-related article is a stub. You can help Misplaced Pages by expanding it. |