| Revision as of 05:35, 31 July 2011 editArcadian (talk | contribs)163,050 edits removed Category:Antineoplastic drugs using HotCat← Previous edit | Revision as of 09:40, 13 August 2011 edit undoCheMoBot (talk | contribs)Bots141,565 edits Updating {{drugbox}} (no changed fields - added verified revid - updated 'ChemSpiderID_Ref', 'DrugBank_Ref', 'ChEMBL_Ref', 'ChEBI_Ref', 'StdInChI_Ref', 'StdInChIKey_Ref', 'ChEBI_Ref') per [[Misplaced Pages:WikiProject Chemicals/Chembox validation|Chem/DrugNext edit → | ||
| Line 1: | Line 1: | ||
| {{drugbox | {{drugbox | ||
| ⚫ | | UNII_Ref = {{fdacite|correct|FDA}} | ||
| | Verifiedfields = changed | |||
| ⚫ | | UNII_Ref = {{fdacite| |
||
| | UNII = FU21S769PF | | UNII = FU21S769PF | ||
| | verifiedrevid = |
| verifiedrevid = 442310797 | ||
| | IUPAC_name = 3,3',3<nowiki>''</nowiki>,3<nowiki>'''</nowiki>-(2,3-dihydroporphyrin-5,10,15,20-tetrayl)tetraphenol | | IUPAC_name = 3,3',3<nowiki>''</nowiki>,3<nowiki>'''</nowiki>-(2,3-dihydroporphyrin-5,10,15,20-tetrayl)tetraphenol | ||
| | image = Temoporfin.png | | image = Temoporfin.png | ||
| Line 12: | Line 11: | ||
| | ATC_supplemental = | | ATC_supplemental = | ||
| | PubChem = 60751 | | PubChem = 60751 | ||
| | DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| | DrugBank = | | DrugBank = | ||
| | KEGG_Ref = {{keggcite|correct|kegg}} | | KEGG_Ref = {{keggcite|correct|kegg}} | ||
Revision as of 09:40, 13 August 2011
Pharmaceutical compound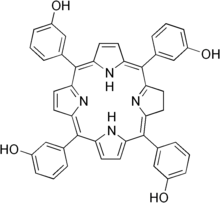 | |
| Clinical data | |
|---|---|
| License data | |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.152.970 |
| Chemical and physical data | |
| Formula | C44H32N4O4 |
| Molar mass | 680.74 g/mol g·mol |
| 3D model (JSmol) | |
SMILES
| |
| (verify) | |
Temoporfin (INN) is a photosensitizer (based on porphyrin) used in photodynamic therapy for the treatment of squamous cell carcinoma of the head and neck . It is marketed in the European Union under the brand name Foscan. The US FDA deemed Foscan non-approvable in 2000. The EU approved its use in June 2001.
Good results were obtained in 21 of 35 patients treated in Germany.
It is photoactivated at 652 nm i.e. by red light.
Patients can remain photosensitive for several weeks after treatment.
Further reading
References
- Lorenz KJ, Maier H (2008). "". HNO (in German). 56 (4): 402–9. doi:10.1007/s00106-007-1573-1. PMID 17516041.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ O'Connor, Aisling E, Gallagher, William M, Byrne, Annette T (2009). "Porphyrin and Nonporphyrin Photosensitizers in Oncology: Preclinical and Clinical Advances in Photodynamic Therapy. Photochemistry and Photobiology, Sep/Oct 2009". Photochemistry and Photobiology.
{{cite news}}: CS1 maint: multiple names: authors list (link) - http://www.highbeam.com/doc/1P2-18794532.html Foscan approval saves Scotia's skin.
- http://www.springerlink.com/content/g74w224824v8l013/
- "Porphyrin and Nonporphyrin Photosensitizers in Oncology: Preclinical and Clinical Advances in Photodynamic Therapy". Photochemistry and Photobiology. 2009.
This antineoplastic or immunomodulatory drug article is a stub. You can help Misplaced Pages by expanding it. |