| Revision as of 10:21, 26 October 2011 edit213.129.160.169 (talk)No edit summary← Previous edit | Revision as of 12:08, 26 October 2011 edit undoBeetstra (talk | contribs)Edit filter managers, Administrators172,031 edits Script assisted update of identifiers for the Chem/Drugbox validation project (updated: 'KEGG', 'CAS_number').Next edit → | ||
| Line 20: | Line 20: | ||
| <!--Identifiers--> | <!--Identifiers--> | ||
| | CAS_number_Ref = {{cascite|changed|??}} | | CAS_number_Ref = {{cascite|changed|??}} | ||
| | CAS_number = 211914-51-1 | | CAS_number = <!-- blanked - oldvalue: 211914-51-1 --> | ||
| | ATC_prefix = <!-- 'none' if uncategorised --> | | ATC_prefix = <!-- 'none' if uncategorised --> | ||
| | PubChem = 6445226 | | PubChem = 6445226 | ||
| Line 30: | Line 30: | ||
| | UNII = I0VM4M70GC | | UNII = I0VM4M70GC | ||
| | KEGG_Ref = {{keggcite|changed|kegg}} | | KEGG_Ref = {{keggcite|changed|kegg}} | ||
| | KEGG = D07144 | | KEGG = <!-- blanked - oldvalue: D07144 --> | ||
| | ChEMBL_Ref = {{ebicite|changed|EBI}} | | ChEMBL_Ref = {{ebicite|changed|EBI}} | ||
| | ChEMBL = 539697 | | ChEMBL = 539697 | ||
Revision as of 12:08, 26 October 2011
Pharmaceutical compound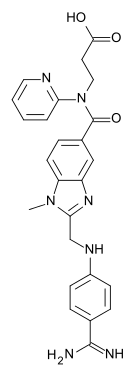 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Consumer Drug Information |
| MedlinePlus | a610024 |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C25H25N7O3 |
| Molar mass | 471.5 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (what is this?) (verify) | |
{{drugbox
| verifiedrevid = 407639691
| drug_name = Dabigatran etexilate
| IUPAC_name = Ethyl 3-{ carbamimidoyl}phenyl)amino]methyl}-1-
methyl-1H-benzimidazol-5-yl)carbonyl] (2-pyridinyl)amino}propanoate
| image = Dabigatran etexilate structure.svg
| width = 135
| ChemSpiderID_Ref =
| ChemSpiderID = 4948999
| InChI = 1/C34H41N7O5/c1-4-6-7-10-21-46-34(44)39-32(35)24-12-15-26(16-13-24)37-23-30-38-27-22-25(14-17-28(27)40(30)3)33(43)41(20-18-31(42)45-5-2)29-11-8-9-19-36-29/h8-9,11-17,19,22,37H,4-7,10,18,20-21,23H2,1-3H3,(H2,35,39,44)
| smiles = O=C(OCC)CCN(c1ncccc1)C(=O)c4ccc2c(nc(n2C)CNc3ccc(C(=N\C(=O)OCCCCCC)\N)cc3)c4
| InChIKey = KSGXQBZTULBEEQ-UHFFFAOYAL
| ChEMBL_Ref =
| ChEMBL = 539697
| StdInChI_Ref =
| StdInChI = 1S/C34H41N7O5/c1-4-6-7-10-21-46-34(44)39-32(35)24-12-15-26(16-13-24)37-23-30-38-27-22-25(14-17-28(27)40(30)3)33(43)41(20-18-31(42)45-5-2)29-11-8-9-19-36-29/h8-9,11-17,19,22,37H,4-7,10,18,20-21,23H2,1-3H3,(H2,35,39,44)
| StdInChIKey_Ref =
| StdInChIKey = KSGXQBZTULBEEQ-UHFFFAOYSA-N
| CAS_number = 211915-06-9
| ATC_prefix = B01
| ATC_suffix = AE07
| ATC_supplemental=
| PubChem = 6445226
| DrugBank =
| chemical_formula =
| C=34 | H=41 | N=7 | O=5
| molecular_weight = 627.734 g/mol
(471.511 without etexilate)
| specific_rotation =
| sec_combustion =
| bioavailability = 3–7%
| protein_bound = 35%
| metabolism =
| elimination_half-life = 12–17 hours
| excretion =
| pregnancy_AU =
| pregnancy_US = C
| pregnancy_category =
| legal_AU =
| legal_CA = Schedule VI
| legal_UK = POM
| legal_US = Rx-only
| legal_status =
| dependency_liability =
| routes_of_administration = oral
| licence_EU = Pradaxa
| licence_US = Dabigatran
}}
Dabigatran (Pradaxa in Australia, Europe and USA, Pradax in Canada, Prazaxa in Japan) is an oral anticoagulant from the class of the direct thrombin inhibitors. It is being studied for various clinical indications and in some cases it offers an alternative to warfarin as the preferred orally administered anticoagulant ("blood thinner") since it does not require frequent blood tests for international normalized ratio (INR) monitoring while offering similar results in terms of efficacy. There is no specific way to reverse the anticoagulant effect of dabigatran in the event of a major bleeding event, unlike warfarin. It was developed by the pharmaceutical company Boehringer Ingelheim.
Development
Dabigatran (then compound BIBR 953) was discovered from a panel of chemicals with similar structure to benzamidine-based thrombin inhibitor α-NAPAP (N-alpha-(2-naphthylsulfonylglycyl)-4-amidinophenylalanine piperidide), which had been known since the 1980s as a powerful inhibitor of various serine proteases, specifically thrombin, but also trypsin. Addition of a hydrophobic side chain led to the orally absorbed prodrug, BIBR 1048 (dabigatran etexilate).
Dosing
A 2004 study showed a good safety profile at doses between 12.5 and 300 mg twice daily.
A phase II study, comparing dabigatran with enoxaparin, showed increased efficacy in preventing thrombosis in patients undergoing orthopedic surgery, but a possible increased bleeding risk in patients receiving higher doses of dabigatran. A phase III study, comparing dabigatran doses of 150 mg and 220 mg once daily with the standard 40 mg dose of enoxaparin once daily, confirmed that dabigatran performed as well as enoxaparin in preventing thrombosis, with a similar risk profile.
Fatty-foods delay the absorption of dabigatran, however the bio-availability of the drug is unaffected. One study showed that absorption may be moderately decreased if taken with a proton pump inhibitor. Drug excretion through p-glycoprotein pumps is slowed in patients taking strong p-gp pump inhibitors such as quinidine, verapamil, and amiodarone, thus raising plasma levels of dabigatran.
Major trials
RE-LY study
A manufacturer-sponsored phase III study, RE-LY, evaluated the efficacy and safety of two different doses of dabigatran relative to warfarin in over 18,000 patients with atrial fibrillation. 18,113 patients with atrial fibrillation were randomized to one of three arms: (1) adjusted dose warfarin, (2) dabigatran 110 mg twice daily, or (3) dabigatran 150 mg twice daily. The warfarin arm was open label, but adverse events were adjudicated by reviewers blinded to treatment. Dabigatran 110 mg was non-inferior to warfarin for the primary efficacy endpoint of stroke or systemic embolization, while dabigatran 150 mg was significantly more effective than warfarin or dabigatran 110 mg. Major bleeding occurred significantly less often with dabigatran 110 mg than warfarin; dabigatran 150 mg showed similar bleeding to warfarin.
Data released in May 2011 show that patients under 75 with atrial fibrillation at risk for stroke have lower risks of both intracranial and extracranial bleeding in both doses of dabigatran compared with warfarin. In patients over 75 years, intracranial bleeding risk is lower but extracranial bleeding risk is similar or higher with both doses of dabigatran when compared with warfarin.
RE-COVER
A 2009 large (2539 patients), randomized, double-blind trial by the RE-COVER study group demonstrated non-inferiority of dabigatran when compared to warfarin in the treatment of acute venous thromboembolism, with a similar rate of major bleeding and a lower rate of combined major plus non-major bleeding. Patients randomized to dabigatran had fewer minor bleeds but more dyspepsia and more drug discontinuation. Dabigatran-treated patients did not undergo coagulation testing.
Approval and indications
On March 18, 2008, the European Medicines Agency granted marketing authorisation for Pradaxa for the prevention of thromboembolic disease following hip or knee replacement surgery and for non-valvular atrial fibrillation.
The National Health Service in Britain authorised the use of dabigatran for use in preventing blood clots in hip and knee surgery patients. The British Heart Foundation is campaigning for the drug to be widely prescribed in place of warfarin, which has the disadvantage of requiring administration up to a week before a target INR level is reached, and heparin, which is administered intravenously or subcutaneously in its low molecular weight form. Dabigatran will cost the NHS £4.20 per day, which is equivalent to several other anticoagulants, but more than ten times the cost of warfarin. However, the total cost of warfarin use includes the time and cost of INR monitoring which is not required with dabigatran.
Pradax received a Notice of Compliance (NOC) from Health Canada on June 10, 2008, for the prevention of blood clots in patients who have undergone total hip or total knee replacement surgery. Approval for atrial fibrillation patients at risk of stroke came in October 2010.
The U.S. Food and Drug Administration (FDA) approved Pradaxa on October 19, 2010, for prevention of stroke in patients with non-valvular atrial fibrillation. The approval came after an advisory committee recommended the drug for approval on September 20, 2010 although caution is still urged by reviewers.
On February 14, 2011, the American College of Cardiology Foundation and American Heart Association added dabigatran to their guidelines for managment of non-valvular atrial fibrillation with a class I recommendation.
Expiration of capsules
Once a bottle of dabigatran is opened, the medication expires after only thirty days. This unusually short period exists because the drug can be affected by humidity. Data currently under review by the FDA indicate that dabigatran maintains its potency up to sixty days after bottle opening as long as it is stored in the original bottle and the handling requirements are met. Blisterpacks do not have that same thirty-day expiration.
References
- ^ Pradaxa Full Prescribing Information. Boehringer Ingelheim. October 2010.
- Hauel NH, Nar H, Priepke H, Ries U, Stassen JM, Wienen W (2002). "Structure-based design of novel potent nonpeptide thrombin inhibitors". J Med Chem. 45 (9): 1757–66. doi:10.1021/jm0109513. PMID 11960487.
{{cite journal}}: Unknown parameter|laysummary=ignored (help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Eriksson BI, Dahl OE, Ahnfelt L; et al. (2004). "Dose escalating safety study of a new oral direct thrombin inhibitor, dabigatran etexilate, in patients undergoing total hip replacement: BISTRO I". J. Thromb. Haemost. 2 (9): 1573–80. doi:10.1111/j.1538-7836.2004.00890.x. PMID 15333033.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Eriksson BI, Dahl OE, Büller HR; et al. (2005). "A new oral direct thrombin inhibitor, dabigatran etexilate, compared with enoxaparin for prevention of thromboembolic events following total hip or knee replacement: the BISTRO II randomized trial". J. Thromb. Haemost. 3 (1): 103–11. doi:10.1111/j.1538-7836.2004.01100.x. PMID 15634273.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Eriksson BI, Dahl OE, Rosencher N; et al. (2007). "Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomised, double-blind, non-inferiority trial". Lancet. 370 (9591): 949–56. doi:10.1016/S0140-6736(07)61445-7. PMID 17869635.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Stangier J, Eriksson BI, Dahl OE; et al. (2005). "Pharmacokinetic profile of the oral direct thrombin inhibitor dabigatran etexilate in healthy volunteers and patients undergoing total hip replacement". J Clin Pharmacol. 45 (5): 555–63. doi:10.1177/0091270005274550. PMID 15831779.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - "Pradaxa Summary of Product Characteristics". European Medicines Agency.
- ^ Connolly, SJ; Ezekowitz, MD; Yusuf, S; et al. (2009). "Dabigatran versus warfarin in patients with atrial fibrillation" (PDF). N Engl J Med. 361 (12): 1139–51. doi:10.1056/NEJMoa0905561. PMID 19717844.
{{cite journal}}: Unknown parameter|month=ignored (help) - "Breakthrough therapy dabigatran provides consistent benefit across all atrial fibrillation types and stroke risk groups". Boehringer Ingelheim. Ingelheim, Germany. 4 April 2011.
- Eikelboom, JW (31 May 2011). "Risk of Bleeding With 2 Doses of Dabigatran Compared With Warfarin in Older and Younger Patients With Atrial Fibrillation: An Analysis of the Randomized Evaluation of Long-Term Anticoagulant Therapy (RE-LY) Trial". Circulation. 123 (21): 2363–72. doi:10.1161/CIRCULATIONAHA.110.004747. PMID 21576658.
- Schulman S, Kearon C, Kakkar AK; et al. (2009). "Dabigatran versus warfarin in the treatment of acute venous thromboembolism" (PDF). N Engl J Med. 361 (24): 2342–52. doi:10.1056/NEJMoa0906598. PMID 19966341.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - "Pradaxa EPAR". European Medicines Agency. Retrieved 2011-01-30.
- "Clot drug 'could save thousands'". BBC News Online. 2008-04-20. Retrieved 2008-04-21.
- "Summary Basis of Decision (SBD): Pradax" Health Canada. 2008-11-06.
- Kirkey, Sharon (29 October 2010). "Approval of new drug heralds 'momentous' advance in stroke prevention". Montreal Gazette. Retrieved 29 October 2010.
- "Pradax (Dabigatran Etexilate) Gains Approval In Canada For Stroke Prevention In Atrial Fibrillation" Medical News Today. 28 October 2010.
- Turpie AG (2008). "New oral anticoagulants in atrial fibrillation". Eur Heart J. 29 (2): 155–65. doi:10.1093/eurheartj/ehm575. PMID 18096568.
{{cite journal}}: Unknown parameter|month=ignored (help) - "Boehringer wins first US OK in blood-thinner race". Thomson Reuters. 2010-10-19. Retrieved 2010-10-20.
- "FDA approves Pradaxa to prevent stroke in people with atrial fibrillation". U.S. Food and Drug Administration (FDA). 2010-10-19.
- Shirley S. Wang (2010-09-20). "New Blood-Thinner Recommended by FDA Panel". The Wall Street Journal. Retrieved 2010-10-20.
- Merli G, Spyropoulos AC, Caprini JA (August 2009). "Use of emerging oral anticoagulants in clinical practice: translating results from clinical trials to orthopedic and general surgical patient populations". Ann Surg. 250 (2): 219–28. doi:10.1097/SLA.0b013e3181ae6dbe. PMID 19638915.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Wann LS, Curtis AB, Ellenbogen KA, Estes NA, Ezekowitz MD, Jackman WM, January CT, Lowe JE, Page RL, Slotwiner DJ, Stevenson WG, Tracy CM, Jacobs AK (2011). "2011 ACCF/AHA/HRS Focused Update on the Management of Patients With Atrial Fibrillation (Update on Dabigatran): A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines". Circulation. 123 (10): 1144–50. doi:10.1161/CIR.0b013e31820f14c0. PMID 21321155.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - "FDA Drug Safety Communication: Special storage and handling requirements must be followed for Pradaxa (dabigatran etexilate mesylate) capsules". U.S. Food and Drug Administration (FDA). 2011-03-29. Retrieved 2011-03-29.
Although the current Pradaxa label states that the product should be discarded 30 days after the original bottle is opened, data currently under review by the FDA indicate that the product maintains its potency up to 60 days after bottle opening as long as it is stored in the original bottle and the handling requirements are met--including that the cap is closed tightly after each use, and the bottle is kept away from excessive moisture, heat, and cold. The manufacturer is gathering more information on whether the product can be used after 60 days and this information will be added to the Pradaxa label when FDA's review is complete.
External links
- Pradaxa.com. Boehringer Ingelheim.
- dabigatran.com. Boehringer Ingelheim.
- Pradaxa For U.S. Health Care Professionals. Boehringer Ingelheim.
- Pradaxa Prescribing Information. Boehringer Ingelheim.
- Pradaxa Medication Guide. Boehringer Ingelheim.
- Dabigatran. MedlinePlus. United States National Library of Medicine (NLM).
- Dabigatran. Drug Information Portal. United States National Library of Medicine (NLM).
| Antithrombotics (thrombolytics, anticoagulants and antiplatelet drugs) (B01) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antiplatelet drugs |
| ||||||||||||||
| Anticoagulants |
| ||||||||||||||
| Thrombolytic drugs/ fibrinolytics | |||||||||||||||
| Non-medicinal | |||||||||||||||
| |||||||||||||||