This is the current revision of this page, as edited by Ozzie10aaaa (talk | contribs) at 00:01, 31 July 2024 (Cleaned up using AutoEd). The present address (URL) is a permanent link to this version.
Revision as of 00:01, 31 July 2024 by Ozzie10aaaa (talk | contribs) (Cleaned up using AutoEd)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)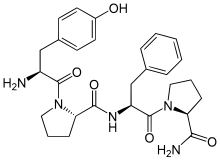
| |
| Names | |
|---|---|
| IUPAC name (2S)-1--N--1-oxo-3-phenylpropan-2-yl]pyrrolidine-2-carboxamide | |
| Other names Tyr-Pro-Phe-Pro-NH2, PLO17 | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C28H35N5O5 |
| Molar mass | 521.6 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Morphiceptin is a tetrapeptide (Tyr-Pro-Phe-Pro-NH2) that is a selective μ-opioid receptor agonist. It is derived from β-casomorphin and has over 1,000 times selectivity for μ- over δ-opioid receptors. When injected intracerebroventricularly (into the ventricular system of the brain), morphiceptin had an analgesic ED50 of 1.7 nmol per animal. The analgesic effects of morphiceptin were reversed by naloxone, meaning that the analgesic effect is mediated by the μ-opioid receptor.
Morphiceptin is the (1S,2S,3S,4S)-form whereas deproceptin is the (1S,2S,3S,4R)-form .
See also
References
- "Morphiceptin". ChemBase. Archived from the original on 15 March 2012. Retrieved 1 August 2011.
- Chang, K (3 May 1982). "Analgesic activity of intracerebroventricular administration of morphiceptin and β-casomorphins: Correlation with the morphine (μ) receptor binding affinity". Life Sciences. 30 (18): 1547–1551. doi:10.1016/0024-3205(82)90242-9. PMID 6281604.