| |
| Names | |
|---|---|
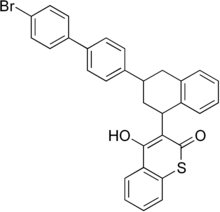
| Preferred IUPAC name 3--4-yl)naphthalen-1-yl]-4-hydroxy-2H-1-benzothiopyran-2-one | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.118.383 |
| KEGG | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C31H23BrO2S |
| Molar mass | 539.49 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Difethialone is an anticoagulant used as a rodenticide. It is considered a second generation agent.
In May 2008, the EPA added restrictions on the sale of difethialone in consumer-use rodenticide products and also for exterior use by commercial applicators.
References
- Nahas K, Lorgue G, Mazallon M (1989). "Difethialone (LM-2219): a new anticoagulant rodenticide for use against warfarin-resistant and -susceptible strains of Rattus norvegicus and Mus musculus". Annales de Recherches Vétérinaires. 20 (2): 159–64. PMID 2751229.
- Saravanan K, Kanakasabai R, Thiyagesan K (June 2003). "Field evaluation of difethialone, a new second generation anticoagulant rodenticide in the rice fields". Indian Journal of Experimental Biology. 41 (6): 655–8. PMID 15266918.
- EPA, OCSPP, OPP, US (2014-03-04). "Restrictions on Rodenticide Products". www.epa.gov.
- "Regulations.gov". www.regulations.gov.
| Pest control: Rodenticides | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Anticoagulants / Vitamin K antagonists |
| ||||||||
| Convulsants | |||||||||
| Calciferols | |||||||||
| Inorganic compounds | |||||||||
| Organochlorine | |||||||||
| Organophosphorus | |||||||||
| Carbamates | |||||||||
| Others | |||||||||
This article about an organic compound is a stub. You can help Misplaced Pages by expanding it. |