 | |
| Clinical data | |
|---|---|
| ATC code | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.047.128 |
| Chemical and physical data | |
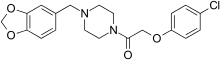
| Formula | C20H21ClN2O4 |
| Molar mass | 388.85 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Fipexide (Attentil, Vigilor) is a psychoactive drug of the piperazine chemical class which was developed in Italy in 1983. It was used as a nootropic drug in Italy and France, mainly for the treatment of senile dementia, but is no longer in common use due to the occurrence of rare adverse drug reactions including fever and hepatitis. Fipexide is similar in action to other nootropic drugs such as piracetam and has a few similarities in chemical structure to centrophenoxine. Chemically, it is an amide union of parachlorophenoxyacetate and methylenedioxybenzylpiperazine (MDBZP), and has been shown to metabolize to the latter, which plays a significant role in its effects.
Synthesis

PTC alkylation of piperazine (1) with 2 equivalents of piperonyl chloride (2) in the presence of cetrimonium bromide gives 1,4-bis-piperonylpiperazine (3). Base catalyzed treatment with 4-Chlorophenoxyacetic acid (4) displaces one of the piperonyl groups to give fipexide (5).
See also
References
- Missale C, Pasinetti G, Govoni S, Spano PF, Trabucchi M (February 1983). "". Bollettino Chimico Farmaceutico (in Italian). 122 (2): 79–85. PMID 6871040.
- Bompani R, Scali G (1986). "Fipexide, an effective cognition activator in the elderly: a placebo-controlled, double-blind clinical trial". Current Medical Research and Opinion. 10 (2): 99–106. doi:10.1185/03007998609110426. PMID 3519097.
- Guy C, Blay N, Rousset H, Fardeau V, Ollagnier M (1990). "". Therapie (in French). 45 (5): 429–31. PMID 2260037.
- Gardini, G. P.; Palla, G.; Scapini, G.; Cesaroni, M. R. (2006). "Convenient Synthesis of N-Benzyl-N′-acyl-piperazines". Synthetic Communications. 12 (11): 887–890. ISSN 0039-7911. doi:10.1080/00397918208065967.
- Anon., FR 7524M (1969-12-15).
- Gian P. Gardini, Giancarlo Scapini, Armando Raimondi, Placido Poidomani, U.S. patent 4,225,714 (1980 to Farmaceutici Geymonat Sud S.P.A.).